In a decision of 21 March 2012, the Cour d’Appel of Paris ruled on the issue of the appraisal which the French judge has to make when a request for a preliminary injunction against acts allegedly infringing a patent or an SPC is referred to him.
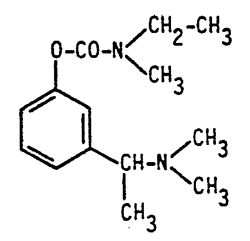
Novartis AG is the holder and SAS Novartis Pharma is the licensee of French patent FR 88 02 597 and of SPC No. 98C0033, both relating to “Rivastigmine”, which is a phenyl carbamate with anticholinesterase activity useful in the treatment of senile dementia.
Rivastigmine is marketed by SAS Novartis Pharma in France, under the name Exelon, for the symptomatic treatment of Alzheimer’s disease and Parkinson’s-related dementia.
The rights on SPC No. 98C0033 expire on 31 July 2012.
Since October 2010, Novartis AG and Novartis Pharma (hereinafter referred to as “Novartis”) had become aware that Mylan and Qualimed were carrying out the necessary formalities to place on the French market generic drugs of the proprietary drug Exelon (1.5, 3, 4.5, 6 mg, capsule):
- On 1 and 4 October 2010, the Agence Française de Sécurité Sanitaire des Produits de Santé (AFSSAPS) granted Mylan and Qualimed the marketing authorisation (MA) for the proprietary drugs named “Rivastigmine Qualimed” and “Rivastigmine Mylan” (1.5, 3, 4.5, 6 mg, capsule);
- These MAs were registered in the index of generic drugs by way of a decision of the AFSSAPS dated 23 December 2010;
- Mylan and Qualimed filed an application with the Comité Économique des Produits de Santé (CEPS) for the registration of their proprietary drugs “Rivastigmine Qualimed” and “Rivastigmine Mylan”;
- On 17 February 2011, the CEPS informed Novartis Pharma that Mylan, which is the owner of Qualimed, had indicated that it “could market its generic drugs without infringing Novartis’ rights over SPC No. 98C0033” within six months following their registration in the Official Journal;
- The drugs Rivastigmine Qualimed and Rivastigmine Mylan were registered in the list of refundable medicinal products on 29 April 2011.
Consequently, on 17 March 2011, Novartis summoned Mylan and Qualimed to appear before the Judge ruling in preliminary proceedings of the Tribunal de Grande Instance of Paris, requesting, an order enjoining them from infringing patent FR 597 and SPC No. 98C0033 pursuant to Article L. 615-3 of the French Intellectual Property Code.
According to Article L. 615-3, as amended by the 29 October 2007 Act, implementing Article 9 of Directive (EC) No. 2004/48, any person with authority to bring an action for infringement may request that the Judge ruling in preliminary proceedings order any measure aimed at preventing an imminent infringement of its rights or aimed at putting a stop on allegedly infringing acts.
|
Article L. 615-3 of the French Intellectual Property Code “Any person with authority to bring an action for infringement may, in preliminary proceedings, request the competent civil court to order, under a penalty of a daily fine if necessary, against the alleged infringer or intermediaries whose services it uses, any measure aimed at preventing an infringement about to be committed against rights conferred by the title or aimed at stopping any further allegedly infringing act. […]The court, in preliminary or ex parte proceedings, may order the requested measures only if evidence, reasonably accessible to the claimant, make it likely that its rights are infringed or that such infringement is about to be committed”. |
In this particular case, the claimants precisely wanted to prevent an imminent infringement of their rights and they requested, pursuant to Article L. 615-3, that the two competitors be enjoined from importing and/or manufacturing, holding, using, offering for sale and selling, and more generally from marketing the drugs Rivastigmine Mylan and Rivastigmine Qualimed (1.5, 3, 4.5 and 6 mg, capsules), under this name or any other name.
However, by way of an order handed down on 21 June 2011 after hearing all the parties, the Judge ruling in preliminary proceedings of the Tribunal de Grande Instance of Paris dismissed Novartis’ claims against Mylan and Qualimed mainly on the grounds of the dispute relating to the lack of inventive step, based on some prior art documents, likely to deprive of validity patent FR 597, whose claimed protection extends to SPC No. 98C0033.
Novartis then lodged an appeal against this decision.
It set out that, pursuant to Article L. 615-3, the preliminary injunction is subject to the likelihood of the infringement. If the patent seems obviously invalid to him, the Judge ruling in preliminary proceedings is certainly right in deeming the infringement not likely. However, this obviousness of the patent invalidity was not the criteria or the condition held in the order, as the first instance Judge thought he could based his decision on the likelihood of a lack of validity of Novartis’ patent. However, the mere likelihood of a lack of validity is not enough to dismiss the claim based on Article L. 615-3 since the Judge ruling in preliminary proceedings, who is also referred to as the “juge de l’apparence” (judge ruling on appearances) or “juge de l’évidence” (judge ruling on obviousness), does not rule on the merits of the case but only renders an interim decision based on the elements that are brought before him, and therefore does not have at his disposal all the necessary elements to decide whether the patent in dispute should be held valid or not. Therefore, Novartis asserted that the appealed order contained a “serious error” on this issue.
It also set out that, in the present case, the likelihood of the infringement of Novartis’ patent and SPC was neither disputed nor disputable since the generic drugs of Mylan and Qualimed were, by definition, copies of the patented drug. Nor was there any doubt or real dispute as to the imminence of the infringement before the first instance Judge.
Novartis also developed arguments to dispute the invalidity of its patent because of a lack of inventiveness. In turn, Mylan and Qualimed requested that the Cour d’Appel of Paris affirm the appealed order and developed arguments according to which the basic patent was invalid.
In its 21 March 2012 decision, the Cour d’Appel of Paris accedes to Novartis’ reasoning about the condition for initiating preliminary injunction proceedings provided for by Article L. 615-3.
The procedure provided for by Article L. 615-3 is autonomous and the conditions for its application differ from those set by Articles 808 and 809 of the French Code of Civil Procedure concerning general preliminary proceedings.
Article L. 615-3, as amended by the 29 October 2007 Act, merely subordinated its measures “to the likelihood of the infringement of the protected rights and not to the likelihood of the validity of the patent from which they derive”. And “before the Judge ruling in preliminary proceedings, Judge ruling on obviousness, only the obvious invalidity of the title can make it unlikely that these rights are about to be infringed”.
In this case, there was precisely such a likelihood that Novartis’ patent and SPC were about to be infringed and there was no obvious invalidity of patent FR 597, whose claimed protection extends to SPC No. 98C0033:
- Mylan and Qualimed’s generic drugs were, by definition, copies of the patented drug and, consequently, potentially infringing products (“it is not disputed that these drugs, which constitute generic drugs, i.e. products that are drugs having the same qualitative and quantitative compositions in active ingredients as the reference drug as well as the same pharmaceutical form, copy the drug covered by SPC No. 98C0033, whose rights benefit Novartis and expire on 31 July 2012”);
- The mere formalities carried out by Mylan and Qualimed before the AFSSAPS and the CEPS could not demonstrate an imminent infringement. The French legislator had expressly authorised generic manufacturers to carry out all the necessary formalities to place on the market their products before the extinguishment of the intellectual property rights over the proprietary drug (Article L. 5121-10 of the French Public Health Code; see also Article L. 613-5 d) of the French Intellectual Property Code). However, the declaration of Mylan, before the CEPS, that it “could market its generic drugs without infringing Novartis’ rights over SPC No. 98C0033” within six months following their registration in the Official Journal, assuredly demonstrated sufficiently that the infringement was imminent;
- While only the obvious invalidity of the title can make it unlikely that the protected rights are about to be infringed, such was not the case of Novartis’ patent FR 597 whose claimed protection extends to SPC No. 98C0033. Unless he was setting himself up as a scientist, the Judge ruling in preliminary proceedings, in view of his powers, could not consider as obvious all of Mylan and Qualimed’s arguments against the inventiveness while the respondents themselves underline the necessary interpretation of scientific documents and analyses. We must agree with this idea, already stated, for example, by the Cour de cassation in another context (Civ. 1re, 11 July 2006, No. 03-19838): by definition, what needs interpretation is not obvious.
The Cour d’Appel of Paris finally underlines the practical legitimacy of its solution which dismisses the claim for a preliminary injunction against an imminent infringement only on the grounds of the obvious invalidity of the title.
Although they had been granted the MA for their generic drug on 1 and 4 October 2010, had decided to market it approximately fifteen months before the expiry of the SPC in issue, and although patent FR 597 had been filed for approximately twenty years, Mylan and Qualimed waited until the last moment, i.e. the day after the summons to appear in preliminary proceedings which had been served upon them by Novartis (17 March 2011), to serve a summons upon Novartis in order to note the invalidity of patent FR 597 for lack of novelty or inventive step.
Therefore, Mylan and Qualimed could only blame themselves if they suffered from the effects of the immediate injunction based on a patent that might be held invalid later by the trial court. The preliminary proceedings are not appropriate to defend themselves by invoking grounds that are not obvious in support of their claim for invalidity.
“Mylan and Qualimed only had to serve the summons for invalidity of the said patent within the time limit allowing them to obtain a judgment on the merits, before proceeding, if necessary, to the marketing of the drug at issue, in order to avoid infringing the rights of the holders of this patent”.
It has some flavour of the “clear the way” concept applied by the English courts (which may be given less weight since the judgement of the High Court in Cephalon v Orchid & Generics (UK) t/a Mylan [2010] EWHC 2945 (Pat)).
Original French decision.
English translation.
Author: Nicolas Bouche, Head Legal Research and Literature, Véron & Associés, Paris, France
________________________
To make sure you do not miss out on regular updates from the Kluwer Patent Blog, please subscribe here.


