A movement is emerging now among the French courts to stay the proceedings in litigations relating to supplementary protection certificates (SPCs) and more precisely relating to the interpretation of Article 3(a) of Regulation (EC) No. 469/2009 (former Regulation No. 1768/92): “the product is protected by a basic patent in force”. These proceedings are stayed waiting for future decisions of the Court of Justice of the European Union (CJEU) to which several questions on that subject have been referred by British courts for a preliminary ruling.
This is first the case of the French Cour de Cassation before which an appeal has been lodged against a 6 November 2009 decision of the Cour d’Appel of Paris (subject-matter of a previous post) rendered to the detriment of the japanese company Daiichi Sankyo (hereinafter referred to as Daiichi). On 10 May 2011, the Cour de Cassation decided to stay the proceedings until the CJEU has handed down a decision on the question referred for a preliminary ruling by way of the orders dated 5 November 2010 (case C-6/11) and 24 June 2010 (case C-322/10) of the Patents Court of England and Wales.
There are at the moment 4 references for a preliminary ruling before the CJEU on the same question of the interpretation of Article 3(a) of Regulation No. 469/2009:
– a reference for a preliminary ruling from Court of Appeal (Civil Division) (England & Wales) made on 5 July 2010 (C-322/10 – Medeva BV v Comptroller-General of Patents),
– a reference for a preliminary ruling from High Court of Justice (Chancery Division) (United Kingdom) made on 27 August 2010 (C-422/10 – Georgetown University, University of Rochester, Loyola University of Chicago v Comptroller-General of Patents, Designs and Trade Marks),
– a reference for a preliminary ruling from High Court of Justice (Chancery Division) (United Kingdom) made on 24 December 2010 (C-630/10 – University of Queensland, CSL Ltd v Comptroller-General of Patents, Designs and Trade Marks) and
– a reference for a preliminary ruling from High Court of Justice (Chancery Division) (United Kingdom) made on 5 January 2011 (C-6/11 – Daiichi Sankyo Company v Comptroller-General of Patents).
On 12 January 2011, an order of the president of the CJEU joined the cases C-322/10 and C-422/10, connected by their object: the interpretation of Article 3(a) and (b) of Regulation (EC) No. 469/2009 of the European Parliament and of the Council of 6 May 2009 concerning the supplementary protection certificate for medicinal products. The opinion of the advocate general on these two joined cases (C-322/10 and C-422/10) should be delivered on 31 July 2011.
But it is also the case of the Cour d’Appel of Paris before which four appeals have been lodged against four decisions of the Director General of the INPI (French Industrial Property Office), refusing the grant of SPCs to the Dutch company Medeva BV (hereinafter referred to as Medeva). On 11 May 2011, the Cour d’Appel of Paris decided to stay these four proceedings until the CJEU and the French Cour de Cassation have handed down a decision in their respective litigations.
All these cases have a same problem in common. The medicinal product subject-matter of the claimed marketing authorisation (MA) is a combination of several active ingredients whereas the product protected by the basic patent only concerns one or some of these active ingredients.
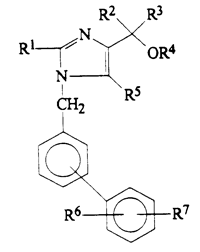
In the case pending before the Cour de Cassation, Daiichi is the holder of a European patent EP 0 503 785 entitled “1-Biphenylimidazole derivatives, their preparation and their therapeutic use” which aims at protecting a series of compounds that have valuable hypotensive activities for the treatment and prophylaxis of hypertension.
And, the claimed MA has been granted in France on 8 February 2006 under No. CIS 66838901 for a proprietary medicine whose active ingredients are two different molecules, i.e. an angiotensin II receptor blocker, olmesartan medoxomil which belongs to the “1-Biphenylimidazole derivatives”, and a diuretic, hydrochlorothiazide which has a molecular structure excluding it from the “1-Biphenylimidazole derivatives”.
Similarly, in the four cases pending before the Cour d’Appel of Paris, Medeva is the holder of the European patent EP 1 666 057 entitled “an acellular vaccine comprising Bordetella pertussis antigens” which aims at protecting a vaccine against whooping cough thanks to antigens, pertactine (69kDa protein) and filamentous haemagglutinin (FHA), specific to the bacterium Bordetella pertussis which causes this disease.
And the medicinal product subject-matter of the claimed MA is, in each case, a multi-disease combination vaccine comprising, on the one hand, the same active ingredients (pertactine and filamentous haemagglutinin) covered by the basic patent and, on the other hand, other active ingredients aiming at protecting against other diseases and therefore not covered by the basic patent (e.g. against diphteria with diphteria toxoid, tetanus with tetanus toxoid, poliomyelitis with inactivated poliovirus, meningitis with haemophilus influenzae).
In all these cases, the grant of the SPC was refused (by the Director General of the INPI or, on appeal, by the Cour d’Appel of Paris) on the same ground that a combination of active ingredients subject-matter of the MA may be the subject-matter of a SPC only if it is protected by the basic patent, what implies that this combination shall be claimed as such in the basic patent. But, precisely, in these different cases, the combination subject-matter of the MA was not protected by the basic patent, in the sense that one or several active ingredients of the combination were not claimed as such in the basic patent. To be protected, the product must be claimed as such. Such a solution implies a literal reading of the claims of the basic patent (“claim test”).
Against this interpretation, Daiichi and Medeva assert that the product “protected by a basic patent”, pursuant to Article 3(a) of Regulation No. 469/2009 of 6 May 2009, is the product falling under the scope of protection of the patent irrespective of whether this product is claimed or not as such in the basic patent. The applicable test would then be the “infringement test”, i.e. to determine if the combination of active ingredients, subject-matter of the MA and of the SPC application, is a product infringing the basic patent.
Such an interpretation may especially rely on the ECJ’s ruling in Farmitalia (C-392/97). ECJ decided that, in order to determine, in connection with Article 3(a), whether a product is protected by a basic patent, reference must be made to the non-Community rules which govern that patent and determine its scope of protection. Yet, it is settled case law, in the field of patents, that patent infringement is established (i.e. the patent confers protection) when the patented element can be found as such among other elements in the same litigious object. Thus, it would be possible to say for example that the basic patent covering anti-hypertensive compounds protects the product containing both such an anti-hypertensive compound and also a diuretic (Daiichi Sankyo’s case), or that the basic patent covering two specific antigens of whooping cough, pertactine (69kDa protein) and filamentous haemagglutinin (FHA), protects the product containing these two specific antigens among several other vaccines against other diseases (Medeva’s cases).
French case law is hesitating on the interpretation of Article 3(a) of Regulation No. 469/2009 (former Regulation No. 1768/92). Some decisions refused the SPC on the basis of a “claim test” (Cour d’Appel of Paris, 4th ch., sect. A, 19 January 2005, Abbot Laboratories; Cour d’Appel of Paris, 4th ch., sect. A, 9 April 2008, Health Research). But the Cour d’Appel of Paris also applied a “disclosure test” in other decisions, granting (Cour d’Appel of Paris, 4th ch., sect. B, 9 December 2005) or refusing the SPC (Cour d’Appel of Paris, 4th ch., sect. A, 8 February 2006, Du Pont de Nemours). To be protected, the product must be disclosed or identifiable in the basic patent (not only the claims but also the description are taken into account).
The hesitation also exists on a international scale as for example Dutch case law had applied a “disclosure test” (OCNL, 7 September 2010, Tocilizumab) and German case law an “infringement test” (Federal Patent Court, 3 Ni 49/07, 16 June 2009, ACE-Inhibitor).
Original French decision (Daiichi Sankyo v. INPI).
English translation (Daiichi Sankyo v. INPI).
Original French decision (Medeva BV v. INPI, one of the four decisions).
Author: Nicolas Bouche, Head Legal Research and Literature, Véron & Associés, Paris, France
________________________
To make sure you do not miss out on regular updates from the Kluwer Patent Blog, please subscribe here.


