The first decisions by the Central Division Munich, the Local Division Paris and the Local Division Düsseldorf adjudging a patent’s validity in main proceedings are now out. Taken together, they confirm the initial favourable impression from applications for provisional measures and demonstrate that this Court is fully up to task. As regards validity, it will take a sober, fresh and critical look at the patents at stake, and it should surprise no one that not all patents will survive the court’s scrutiny. It is too early to draw statistics from two full revocations and one maintenance in part, and many more decisions are currently in the pipeline. Nonetheless, I dare to make the prediction that the overall picture will not be unlike the statistics we see before the Boards of Appeal at the European Patent Office.
The first two (in fact, it is only one in substance) decisions by the Central Division (CD) Munich are particularly instructive and helpful, as they are clearly structured and summarise general points of law in useful headnotes. In the following I will focus on Decision CC_586764/2023 (UPC_14/2023), which is the counterclaim for revocation in the infringement action between Amgen Inc and Regeneron Pharmaceuticals Inc. that Amgen filed on the very first day when the UPC opened its doors (1 June 2023), a few minutes after Sanofi (Regeneron’s licensee) had filed a revocation action at the Central Division, which became the UPC’s very first case ever (UPC_1/2023). The two cases were consolidated before the CD Munich and have now been decided by the Court of First Instance. Amgen’s patent EP 3 666 797 was revoked and Amgen will have to bear attorney fees of €1,375,000 per case on top of that. Of course, both decisions are appealable and, in view of the history of this case and the apparent willingness of both parties to fight this case before the UPC with teeth and claws, I suppose that Amgen will seek redress at the Court of Appeal.
Lesson 1: Claim Interpretation – The Description Matters Always
Headnote 1 summarizes the CD’s general approach on claim interpretation. I let the court speak for itself
1. When interpreting a patent claim, the person skilled in the art does not apply a philological understanding, but determines the technical meaning of the terms used with the aid of the description and the drawings. From the function of the individual features in the context of the patent claim as a whole, it must be deduced which technical function these features actually have individually and as a whole. The patent description may represent a patent ́s own lexicon.
No serious patent practitioner will be very surprised by this headnote, which is firmly based on Art. 69 EPC and the Protocol on Interpretation and fully in line with the established national jurisprudence in the UPC member states, notably with German case law. Each claim feature is susceptible to interpretation, and its technical meaning must be determined with the aid of the description and drawings (if any). The interpretation should be oriented to the function that the individual features have in the context of the patent claim. And, according to the German “Spannschraube” doctrine, the description may represent a patent’s own lexicon, meaning that terms of a claim may be given a different meaning than they usually have, if the description so requires.
The latter approach may have consequences that patent practitioners all over the world should never underestimate. Many practitioners, both in Europe and particularly in the USA, prefer drafting patent applications in the loosest and least limiting way possible. While this may help to secure a broad scope of protection, it has the inevitable consequence that the claim gets vulnerable when its validity is challenged. So better think twice before using boiler plate language and open-ended terms such as “comprising” ad infinitum. For example, if the claim requires the “binding” of an antibody to the “catalytic domain” of a protein named PCSK9, and is supposed to “prevent or reduce” the binding of LDLR to PCSK9 so as to lower blood cholesterol levels, a court may very well consider, in the absence of a narrower definition of “binding” in the description, that the term “binding to the catalytic domain” does not exclude the additional binding to other parts of the protein. And if you write in the description that “prevent or reduce” the binding of LDLR to PCSK9 means the reduction of the quantity of binding partner by at least about 1-20% and up to 98-99% or more, then don’t complain that the UPC (or any other court) will draw the broadest possible inferences against you on validity. A balance will need to be struck carefully.
Lesson 2: Priority will be determined according to the EPO’s “Gold Standard”
The CD Munich put this in its second headnote as follows:
2. A claimed invention is to be considered the “same invention” as meant in Article 87 EPC (priority right) if the skilled person can derive the subject-matter of the claim directly and unambiguously, using common general knowledge, from the previous application as a whole.
and explicitly referred to G2/98 in the reasons of the decision. Again, no big surprise to this extent: I am not aware that any national court in Europe had recently suggested, let alone applied any different standard. The question rather is how exactly the famous words “directly and unambiguously, using common general knowledge” are filled with life. If I understand the decision correctly, the CD Munich would reject any literalism or photographic approach in this regard and instead focus on the skilled person’s technical understanding. This allows the court to be a bit more flexible than the EPO, similar as the German Courts usually are (see the FCJ’s latest decision X ZR 92/23 Mirabegron as a recent example). In the case at issue, the CD Munich did not hold it against the patentee that Fig. 26, which explicitly depicted the sequence of the catalytic domain of PCSK9, was only disclosed in the fourth priority document (P4). The court opined that Fig. 26 does not contain any new information with respect to the amino acid sequence of the catalytic domain of PCSK9 relative to the whole contents of the third priority application (P3) on which Patentee relied. According to the CD Munich, the skilled person does not see Figure 26 as the (sole) definition of the catalytic domain in P4 or the Patent, see reason 7.11:
Rather Figure 26, whilst keeping consistent with the understanding of the pro-, catalytic and V-domains as follows from P3, shows a sequence comparison of the PCSK9 amino acid sequence (“PCSK9parent”) and residues that were mutated in certain PCSK9 variants (“PCSK9mutants”). With respect to the definition of PCSK9 ́s catalytic domain, Figure 26 therefore does not add or change any technical information vis-à-vis the disclosure of P3 nor does it comprise the sole definition of “catalytic domain” in the Patent (see above, 6.16 which reasoning applies mutatis mutandis here).
Lesson 3: The UPC will not apply the Problem-Solution-Approach as developed by the EPO
While the decisions by the CD Munich include a discussion of inventive step in terms of a problem-solution framework, they do not rigorously proceed according to the EPO’s Problem-Solution-Approach, which generally requires the determination of the “closest state of the art” as a first step. In contrast, the UPC merely requires that the analysis starts from a “realistic starting point”, as succinctly stated in Headnote 3:
3. The assessment of inventive step starts from a realistic starting point in the prior art. There can be several realistic starting points. It is not necessary to identify the “most promising” starting point.
It will be interesting to see whether the EPO will now reconsider and modify its Problem-Solution-Approach somewhat. Even though this approach is widely applied at the EPO and provides sensible results in the majority of cases, it appears nowhere in the European Patent Convention and there are also some decisions by Technical Boards of Appeal that do not religiously follow this approach. Conversely, as again the FCJ’s most recent decision Mirabegron shows, German case law has never accepted any preference of a “closest” state of the art and usually applies the concept of a “realistic starting point” that needs some (but not much) justification to avoid the appearance of hindsight. Also in the German jurisprudence, there can be several realistic starting points.
In the case at issue, it seemed uncontroversial that prior art reference “Lagace” was a possible and realistic starting point, hence the Court proceeded from there, even though the EPO had used a different reference (“Graham”) as the closest prior art. While the UPC acknowledged this fact, it was satisfied that Lagace is a realistic starting point for the analysis of obviousness, proceeded from there and indeed found that the invention is obvious in view of Lagace. In the Court’s own words:
Having concluded that Lagace is a realistic starting point, the Central Division does not have to examine in detail whether another starting point, in particular Graham as suggested by the Defendant, is “more promising”. As set out above, the claimed subject matter has to be inventive over any realistic starting point.
Lesson 4: For the UPC, “obvious” is the “next step”
The notion of a next step, which is usually the obvious one, is perhaps a bit “UPC-special”, but the CD Munich decided to follow the Court of Appeal, who applied this concept in the 10x Genomics case, in this regard.
4. In general, a claimed solution is obvious if the skilled person would be motivated to consider the claimed solution and would implement it as a next step in developing the prior art. It may be relevant whether the skilled person would have expected any particular difficulties in taking any next step(s). The absence of a reasonable expectation of success (or more in general: non-obviousness) does not follow from the mere fact that other ways of solving the underlying problem are also suggested in the prior art and/or (would) have been pursued by others. The decisive question that has to be answered is whether the claimed solution is non-obvious.
It remains to be seen how the UPC will decide cases where more than one step is necessary to proceed from the “realistic starting point” to the claimed invention, particularly if there was a motivation to take more than just one step. A motivation seems to be required in any case, which as such is perhaps not so surprising. Of more interest is the Court’s general comment that prior art pointing in a different direction and/or other ways of solving the underlying problem that a skilled person could or would have also considered do not render an solution inventive that is otherwise obvious.
In the case at issue, claim 1 (slightly simplified) pertained to a monoclonal antibody for use in
treating or preventing diseases associated with an elevated cholesterol level, wherein the monoclonal antibody binds to the catalytic domain of a PCSK9 protein of the amino acid sequence of SEQ ID NO: 1, and prevents or reduces the binding of PCSK9 to LDLR. LDLR is the “low density lipoprotein receptor”, which mediates the uptake and degradation of cholesterol-rich lipoproteins in certain cells and thus lowers the blood cholesterol level. PCSK9 in turn acts by decreasing LDLR levels, as shown in the following schematic drawing taken from the decision, 8.48.
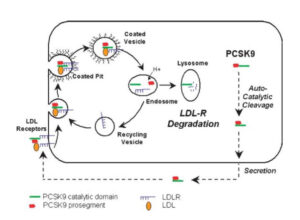
The problem for patentee in this case was that this mechanism was already known and, even worse for patentee, that Lagace also drew the following conclusion therefrom, which the court quoted in its decisions (8.29):
If PCSK9 functions as a secreted factor as suggested by the current data, then additional approaches to neutralize its activity, including the development of antibodies to block its interaction with the LDLR or inhibitors to block its action in plasma, can be explored for the treatment of hypercholesterolemia. (emphasis CD)
The CD acknowledged that Lagace does not disclose any concrete antibodies that bind to the catalytic domain of PCSK9 and block the interaction between PCSK9 and LDLR, nor does it actually use such antibodies for the treatment of hypercholesterolemia. However, it found that implementing this proposal by developing antibodies that block the interaction between PCSK9 and LDLR would have been the next step taken by the skilled person, and that pursuing this route, the skilled person would have ended up with antibodies as claimed without inventive skill.
The decisions by the CD Munich include many more details and explanations, for which I would refer the reader to the original decisions, which are worth reading in full. I will confine myself to just two more short lessons to be learned from this decision.
Lesson 5: Inevitability is not required for Obviousness
For example, patentee argued that it was far from clear that a skilled person would have used antibodies to block PCSK9. Indeed, Graham, a paper from scientists working in this field, did not use antibodies, but antisense single oligonucleotides (ASOs). Thus, the only concrete implementation of an agent blocking PCSK9 did not use antibodies. However, the court was not too impressed by this argument, since antibodies had also been suggested for precisely this purpose by Lagace. Thus, while it would also have been possible for a skilled person to turn to ASOs, this does not render the use of antibodies inventive:
5. For assessing inventive step it is not the question whether the skilled person would inevitably arrive at the same result (falling within the scope of the claim or not). Rather, it is sufficient (but also necessary) for denying inventive step that the skilled person would without inventive contribution arrive at a result which is covered by a claim.
Lesson 6: Features that appear arbitrary do not generally support Inventive Step
There was a debate about which effect was associated with the binding to the catalytic domain of PCSK9 as required by the claim. However, in the court’s view, there was no apparent causal technical connection between the feature “binds to the catalytic domain” and the reduction of the binding of PCSK9/LDRL and, ultimately, the therapeutic effect claimed. The CD Munich therefore was of the opinion that the feature of binding to the catalytic domain cannot contribute to inventive step. The skilled person knew at the relevant date that PCSK9 consisted of three domains. Specifying that the antibodies bind to the catalytic domain as interpreted by the skilled person, is an arbitrary choice out of several possibilities that cannot render the claimed subject matter inventive (8.78).
This is summarized in the following headnote:
6. A technical effect or advantage achieved by the claimed subject matter compared to the prior art may be an indication for inventive step. A feature that is selected in an arbitrary way out of several possibilities cannot generally contribute to inventive step.
Conclusion
Together with the decisions in the 10x Genomics v. Nanostrings cases and a decision by the LD Düsseldorf, the two decisions by the CD Munich set first clear signposts of how the UPC will deal with validity matters. At least experienced EPO and German practitioners will be pleased to learn that there are practically no surprises so far. That the approach on validity taken by the UPC so far closely resembles the approaches taken by the Boards of Appeal of the EPO and by the German courts. This does of course not exclude that differences may emerge from case to case in the future. In any case, the fact that the UPC will not rigidly apply the EPO’s problem-solution-approach is unlikely, at least in my view, to result in vastly different outcomes in practice.
Contrary to what some patentees may have hoped, the new UPC is – at least so far – not more generous or patentee-friendly than national courts or the EPO and certainly has no problem in invalidating patents. In my view, the new court tries to convice users by its speed and by the quality and thoroughness of its decisions rather than by any particular kind of outcome.
________________________
To make sure you do not miss out on regular updates from the Kluwer Patent Blog, please subscribe here.



Thank you for these excellent “first lessons”!
Any idea on why the names of the experts have been redacted?
This decision was commented in my blog. The patent in question was the result of a divisional application.
In that blog post, I pointed out that although the patent had been revoked by the Munich LS of the UPC-CD in all UPC member states, an opposition was still pending before the EPO.
It will therefore be interesting to see whether the EPO and its BAs follow the decision of the Munich LS of the UPC-CD. In the event of contradictory decisions, the situation will become interesting. The parent patent has been maintained in amended form by the EPO.
In any case, it is a safe bet that this UPC decision will be appealed.
Only after this appeal will it be possible to see the UPC’s position on the interpretation of the claims, the validity of the priority (same invention) and the assessment of the inventive step.
Alone the question of interpretation of the claims renders the referral G 1/24 highly relevant
On the issue whether the courts, considering whether claimed subject matter was obvious, is there some “middle ground” between, on the one hand, the court’s adopting in full the EPO’s very prescriptive problem-solution approach and, on the other hand, disapproving the EPO approach? Specifically, how does the court’s adoption in this case of a “realistic starting point” offend or contradict EPO-PSA?
Putting it another way, is there yet any reason to expect that the courts, given the same fact matrix as the EPO, will come to a different conclusion on obviousness? I mean, it’s not as if the UPC is operating under the adversarial fact-finding processes of English law, is it?
When was it ever the case that a court, considering the obviousness issue in a blockbuster no-expense-spared patent dispute over both infringement and validity, had for consideration exactly the same fact matrix as the Patent Office that granted the patent in the first place? Given a different matrix of fact, a different conclusion on obviousness is a possibility, no, even when court and EPO use exactly the same methodology for their respective enquiries into obviousness.
Early days yet though, eh, for the UPC? One hopes that diversity and collegiality within the patent courts of Europe will lead to their developing an obviousness enquiry methodology that is the more rigorous, predictable and fair than anything else on offer, anywhere in the world.
Dear Max Drei,
I do fully agree with your hope. I fear that your hope will never materialise.
Having heard the proponents of the UPC and seen some decisions of the Court of Appeal, it is clear that the UPC strives actively to become the leading patent court in Europe, be it only because it will always decide before the EPO, the latter being hampered by inherent procedural delays, even in case of acceleration of the procedure.
See UPC_CoA_22/2024 refusing a stay in case of parallel litigation before the UPC and opposition at the EPO. In this order the CoA disregarded the acceleration procedure before the EPO.
Point 4 of the headnotes in UPC_CoA_22/2024 is very eloquent as far as the attitude of the UPC is concerned: “the interests of harmonising decisions on the validity of a European patent can be promoted by ensuring that the body that decides last can take the decision of the body that decides first into account in its decision”.
In clear: we decide first, you follow and basta! This is far from being collegial.
if even on the same site of the EPO examination and search results vary a lot from examiner to examiner, how can you expect consistency between the EPO and the UPC? And with which one of the many faces of the EPO?