The so-called proportionality defense according to Sec. 139(1) clause 3 (hereinafter simply “proportionality defense”) is one of the newest provisions of the German Patent Act. It has only been introduced in August 2021 through the Patentrechtsmodernisierungsgesetz, a typical German word monster, of which an informative summary was posted here. The main driver of this proportionality defense was the German car industry, which felt more and more hassled by (software) patents covering electronic components of suppliers which, when enforced, could result in a sudden production stop and enormous damages. Conversely, due to the complexity of computer chips and internet applications, it was nearly impossible to conduct a satisfactory freedom to operate search before ordering and using these components. After some back and forth, the legislator introduced the following new clauses into Section 139(1) Patent Act:
(3) The [injunction] claim is excluded to the extent that enforcing the claim due to the special circumstances of the individual case and the requirements of good faith would lead to disproportionate hardship for the infringer or third parties, not justified by the exclusive right. (4) In this case the infringed party shall receive a reasonable monetary compensation. (5) The claim for damages according to subsection 2 remains unaffected.
As this defense is so new, the decision (4c O 18/21) by the Regional Court of Düsseldorf, which has just been released for publication and is the first one in a pharma case where Sec. 139(1) clause 3 has been invoked, might be of particular interest to patent practitioners. A neutralized version of this decision can be found here.
The case concerned a lawsuit filed by the patent proprietor (Nucana plc.) against companies of the Gilead group for patent infringement of Nucana’s patent EP 2955190 by medicaments containing Gileads drug Sofosbuvir. Sofosbuvir is an effective agent against Hepatitis C Virus (HCV).
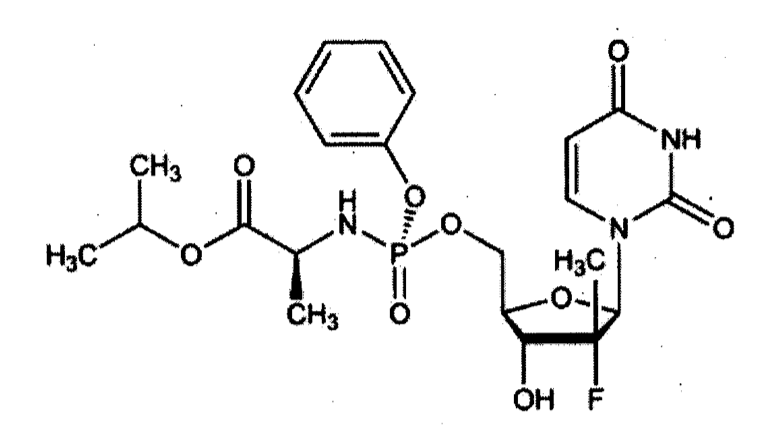
Gilead did not deny that they were infringing the patent but relied on Sec. 139(1) clause 3 in view of patient interests and lack of validity of the patent. The Regional Court dismissed both defences for the following reasons:
(1) The proportionality defense is subsidiary to an action for compulsory license (Sec. 24 Patent Act). If a defendant wishes to rely on this defense, it must primarily bring this case before the Federal Patent Court, which is better equipped to understand and evaluate technical (pharmacological) arguments. Gilead failed on this count because they had only brought their action for a compulsory license a few weeks before the infringement trial.
(2) Moreover, and independently, the proportionality defense is the result of a comprehensive balancing of interests. In addition to third party (patients) interests, the conduct of the parties also needs to be taken into account. Gilead failed on this count because they had, in the Court’s view, not made serious efforts to seek a license from Nucana under appropriate, customary conditions.
(3) On validity, Gilead heavily relied, among others, on the results of a lawsuit filed by Idenix against Gilead, where many courts and the EPO eventually found that Idenix‘ patent was insufficiently disclosed in regard to the synthesis of sofosbuvir. Gilead failed on this count because the Court recognised that the two cases were quite different, both in regard to the disclosure in the underlying patents and in regard to arguments brought before the respective tribunals.
It will be interesting to see what the Higher Regional Court will decide on these counts on appeal. For the time being, however, defendants in a similar situation would be well advised to file their action for compulsory license as early as possible and/or, in the first place, to try and seek a license from the patent proprietor under reasonable conditions.
________________________
To make sure you do not miss out on regular updates from the Kluwer Patent Blog, please subscribe here.


