On 1 June 2021, the Amended Patent Law took effect and introduced patent term extension (“PTE“) and patent linkage (“PL“), which are closely related to the pharmaceutical industry. The legislative amendment reflects China’s determination to promote the research and development of innovative drugs in the pharmaceutical industry. The China National Intellectual Property Administration (“CNIPA“), the National Medical Products Administration (“NMPA“) and the Supreme People’s Court (“SPC“) recently issued further rules to complete the establishment of PL system. As for PTE, as the amended Implementing Rules of the Patent Law (“Amended Implementing Rules“) have not been issued yet, the CNIPA issued some temporary measures to address the issue.
Key takeaways
Patent linkage:
- Only chemical drugs can enjoy the full benefits of PL, though biologics and traditional Chinese medicines are also covered.
- Use patent for biologics is subject to PL.
- China Orange Book – Patent Information Registration Platform for Marketed Drugs is officially launched.
- Both civil action and administrative action are available to address PL disputes over chemical drug related patents, and nine months of waiting period is set.
Patent term extension:
- PTE only applies to innovative drugs approved on and after 1 June 2021.
- A request for PTE in paper form shall be submitted to the CNIPA within three months from the issue date of a market approval by the NMPA, though the CNIPA will only review such request after the implementation of the Amended Implementing Rules.
In depth
1. Patent Linkage
On 4 July 2021, the NMPA and the CNIPA jointly issued Implementing Measures for the Early Resolution Mechanism Drug Patent Disputes (for Trial Implementation) [1]https://www.nmpa.gov.cn/xxgk/ggtg/qtggtg/20210703223942131.html (“Trial Implementing Measures“) which became effective on the same day and presented detailed measures regarding the patent linkage system.
Qualified drugs and patents
According to this Trial Implementing Measures, the following types of drugs and invention patents are eligible for patent linkage:
- for chemical drugs — compound patents for active pharmaceutical ingredients, formulation patents and use patents
- for biologics — sequence structure patents and use patents
- for traditional Chinese medicines — composition patents, patents for herb extracts and use patents
It is worthwhile to point out that use patents for biologics have been newly added to the Trial Implementing Measures, which is a positive step during the establishment of these Measures.
Other types of patents aside from those mentioned above and patents unregistered for patent linkage cannot enjoy the benefits of patent linkage.
It is also worthwhile to note that only chemical drug-related patents can enjoy the full benefits of patent linkage, which we will discuss further below.
China Orange Book – Patent Information Registration Platform for Marketed Drugs
This Trial Implementing Measures stipulate that the Patent Information Registration Platform for Marketed Drugs (“PL Platform“) (.zldj.cde.org.cn) established by the Center for Drug Evaluation (“CDE“) under the NMPA is the foundation of the patent linkage system.
In May 2021, CDE launched the abovementioned PL Platform for testing and allowed market approval (“MA“) holders of existing drugs listed in China to participate until 31 May. Since 4 July 2021, the PL Platform has been made officially available to MA holders of existing and newly approved drugs to register their patents for PL.
The setting of PL Platform includes three portals: Patent Registration, Patent Information Publicity, Patent Certification. Patent Registration portal is used for MA holders to submit and register their patents that are eligible for PL. The required information includes drug details, patent details (particularly the relation between the relevant claims and the drug) and MA’s contact information. According to the Trial Implementing Measures, an MA holder shall register its patents via the PL Platform within 30 days after obtaining an MA for the corresponding drug, and update the information within 30 days if there is a valid change to any of the information.
Patent Information Publicity portal is used for the public, a generic drug [2]For simplicity, generic drugs in this article refer to generics of chemical drugs, biosimilars or same recipes of traditional Chinese medicines applicant in particular, to search for registered patents for a marketed drug.
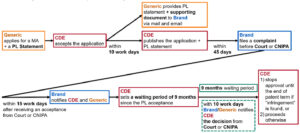
Patent Certification portal is used for CDE to publish a PL statement submitted by generic drug applicants. A generic drug applicant shall submit a PL statement when it submits its application for the generic drug’s market approval. In the PL statement, the generic drug applicant shall check one of the following boxes:
- no patent registered for the brand drug on the PL Platform
- patents registered on the PL Platform had expired or had been invalidated, or a license of the patents is obtained by the generic drug applicant
- the generic drug applicant undertakes not to market the generic drug before the expiry of the patents registered on the PL Platform
- patents registered on the PL Platform should be invalidated, or the generic drug does not fall into the protection scope thereof
The CDE will publish such generic drug applicant’s application and PL statement, and the generic drug applicant shall provide the PL statement and supporting documents to the MA holder, within 10 business days after the MA application had been accepted by the CDE.
MA holders and generic drug applicants are responsible for the authenticity, accuracy and completeness of information they have submitted.
2. Dispute resolution
Patent linkage is established to address the gap between the Bolar Exemption before an MA is issued and patent infringement after the MA is issued. According to the Trial Implementing Measures, a patentee or an interested party (collectively, “Patentee“) is allowed to file a civil action before a court (which is the Beijing IP Court) or an administrative action before the CNIPA (collectively, “PL action“) to address whether the generic drug falls within the protection scope of a registered patent, within 45 days calculated from the date on which the CDE publishes the generic drug MA application. The 45 days would be very tight for foreign patentees to initiate a PL action due to current formality requirements (including notarization and legalization). Thus, it is advisable for foreign patentees to prepare these formality documents in advance.
The Patentee shall inform the CDE and the generic drug applicant within 15 business days after receiving an acceptance notice from the court or the CNIPA, along with a copy of the acceptance notice.
PL civil action
The Supreme People’s Court (“SPC“) released Interpretation for Trial of Civil Cases involving Drug Patent Disputes over Market Approval (“SPC PL Interpretation“) on 5 July 2021.[3]http://www.court.gov.cn/fabu-xiangqing-311791.html The SPC sets forth that all civil lawsuits related to the patent linkage (cases filed under Article 76 of the Amendment of Patent Law, “PL civil action“) shall be heard by the Beijing IP Court. When initiating a PL civil action, the Patentee is required to submit information of the patent registered on the PL Platform, PL statement and supporting documents provided by the generic drug applicant, and prima facie evidence on the generic drug falling into the protection scope of the patent. On the other hand, the generic drug applicant shall submit a copy of the technical documents filed with the CDE, which address the issue of whether it falls into the protection scope of the patent. To ensure the efficiency of the proceeding, the PL civil action will not be stayed due to the initiation of a relevant invalidation action. Further, a declaratory judgement action is available for the generic drug applicant, if the patentee does not initiate a PL action within the abovementioned 45 days. The SPC PL Interpretation confirms that an in-suit preliminary injunction is available for the Patentee in a PL civil action, to obtain interim remedy against the patent infringement conducted by the generic drug applicant. It is worthwhile to note that the SPC PL Interpretation sets forth a rule to protect the generic drug applicant from malicious action. Where the Patentee files a PL action when it knows or should know patents registered on the PL Platform should be invalidated, or the generic drug does not fall into the protection scope thereof, the generic drug applicant is entitled to sue the Patentee before the Beijing IP Court for its loss caused by such action.
PL administrative action
The CNIPA released the Administrative Adjudication Measures for Mechanism of Drug Patent Dispute Resolution (“CNIPA PL Rules“)[4]https://www.cnipa.gov.cn/art/2021/7/5/art_74_160566.html launched on 5 July, 2021.
According to the CNIPA PL Rules, the CNIPA sets up a committee for PL administrative action. The CNIPA PL Rules adopt similar rules as provided in the SPC PL Interpretation. In addition, to ensure the efficiency of the proceeding, the committee may issue a decision based on both parties’ evidence without a hearing.
More details of the SPC PL Interpretation and CNIPA PL Rules in our follow up articles.
For chemical drugs
The CDE will set a one-time waiting period of nine months for chemical generic drugs after receiving the acceptance notice issued by the court or the CNIPA. It is worthwhile to note that the CDE will continue to conduct a technical review in relation to generic drug applications.
The Patentee or the generic drug applicant shall submit an effective judgment/decision (e.g., decision from PL action or invalidation action) issued by the court or the CNIPA, respectively, to the CDE within 10 business days upon the receipt thereof. The CDE may either delay the grant of approval of the generic drug based on the judgment/decision favoring the Patentee or proceed with the grant process.
As incentives to generic drug applicants to proactively challenge relevant registered patents, the CDE shall grant an up to 12-month market exclusivity period (subject to the expiry of a patent) to the first successful patent challenger. To qualify as the first successful patent challenger, a generic drug applicant shall: (a) first successfully challenge the patent; and (b) first receive an MA for the generic drug. During the market exclusivity period, the NMPA will not approve other generic applications for the same drug. It is worthwhile to note that the CDE will continue to review and grant MA applications filed by generic applicants who challenge the patent before the grant of market exclusivity, and to conduct a technical review filed by other applicants.
For biologics or traditional Chinese medicines
Unfortunately, no waiting period is available for potentially disputed generic applications for biologics or traditional Chinese medicines, and the NMPA will decide whether to grant an MA upon the completion of a relevant technical review regardless whether there is a PL action.
In summary, we create a flow diagram below to demonstrate the patent linkage system according to the Trial Implementing Measures.
3. Patent term extension
According to Article 42.3 of the Amended Patent Law, upon the request of a patentee, an invention patent may obtain a term extension of up to five years and enjoy a total patent term of up to 14 years after market approval is issued to a related novel drug in China, and the abovementioned calculation of the term extension which combines the methods of the EU and the United States.
However, the definition of a novel drug and the scope of qualified patents are not clear in the above law. We still consider the early draft of the Amended Implementing Rules as a good reference to address these issues; a novel drug would likely refer to one approved for the first time in China rather than globally, and invention patents are limited to product, process and use patents related to the active pharmaceutical ingredients of a chemical drug, a biologic drug and traditional Chinese medicine. Further, it is likely that a patentee can only request PTE for one patent per drug, if there are multiple qualified patents for the drug, and only one drug’s MA can be used to calculate PTE if there are multiple qualified drugs covered by one patent. As for the remaining term of patent, it shall not be less than six months to claim the patent term extension.
On 25 May 2021, the CNIPA issued an administrative rule named “Interim Measures for the Handling of Relevant Examination Procedures upon Implementation of the Amended Patent Law”[5]http://www.cnipa.gov.cn/art/2021/5/25/art_74_159631.html (“Interim Measures“), which took effect on 1 June 2021. On 27 May 2021, the CNIPA officially clarified in a Q&A section that PTE only applies to innovative drugs approved on and after 1 June 2021.
According to the above Interim Measures, a patentee may submit a request for the patent term extension in paper form within three months from the issue date of an MA by the NMPA, and pay fees in accordance with the relevant notice issued by the CNIPA. However, the CNIPA makes it clear that it will only review such application after the implementation of the Amended Implementing Rules.
Though it is unclear when the Amended Implementing Rules will be released, it is advisable to submit a request for PTE based on a product/use patent that has a long remaining patent term within three months of the issuance of an MA and avoid a patent from covering multiple drugs.
Conclusion
The set-up of the patent linkage system and PTE brought about by the Patent Law Amendments is a milestone in the patent regulatory framework in China. The system will impact both branded drugs and generic drugs in the pharmaceutical industry. Pharmaceutical companies should be prepared for disputes related to patent linkage, which would in turn shape the further development of the linkage system.
________________________
To make sure you do not miss out on regular updates from the Kluwer Patent Blog, please subscribe here.

References

