As of February 2021, it is still too early to have a clear and complete view of how the COVID-19 health, economic, and social crisis has affected patent filing trends and how innovation specifically dedicated to cope with the present “new normal” situation is the object of patent protection. This pandemic has prompted patent offices to provide companies and investigators with a large number of new web-based services and dedicated policies to cope with logistical and financial problems related to IP operations, as reported in WIPO COVID-19 IP Policy Tracker. Moreover, many websites giving access to patent databases have established dedicated portals to foster research and access to information relevant for developing new technical solutions, such as the PatentScope COVID-19 Index or the patent datasets EPO patent examiners and data analysts have compiled and which can be accessed at the Fighting Coronavirus webpage. These and other patent information search tools may also be used to evaluate to what extent the examination and publication of COVID-19 related patent applications have been accelerated after their filing. This search allows identifying in which countries applicants have shown more interest in getting a patent granted (or at least disclosing own patent-related activities), due to their own perception of the potential financial and strategic importance.
I have co-authored a study (Falciola L. and Barbieri M., ”Searching and Analysing Patent-Relevant Information for Evaluating COVID-19 Innovation”; posted on the SSRN website on 26 January 2021) on the major trends in scientific and patent publications during the first months as of COVID-19’s appearance. The analysis has been performed in patent databases and official registers of the major patent offices worldwide, using a standardized set of keywords, under three main dimensions: the claimed technologies, the type of patent proceedings, and the countries. The data extracted for the period January-August 2020 show that more than 1,000 patent documents explicitly mentioning the infectious agent (SARS-CoV-2) and/or the disease (COVID-19) were already filed and published. These documents concern products or technologies applicable in a range of domains, mainly in diagnostics and therapeutics but also body protection, disinfection, informatics and mechanical devices. It is interesting to compare the dates of some early 2020 events with the filing dates of the earliest published patent applications, which predate even the official WHO declaration of the COVID-19 pandemic. This is illustrated below.
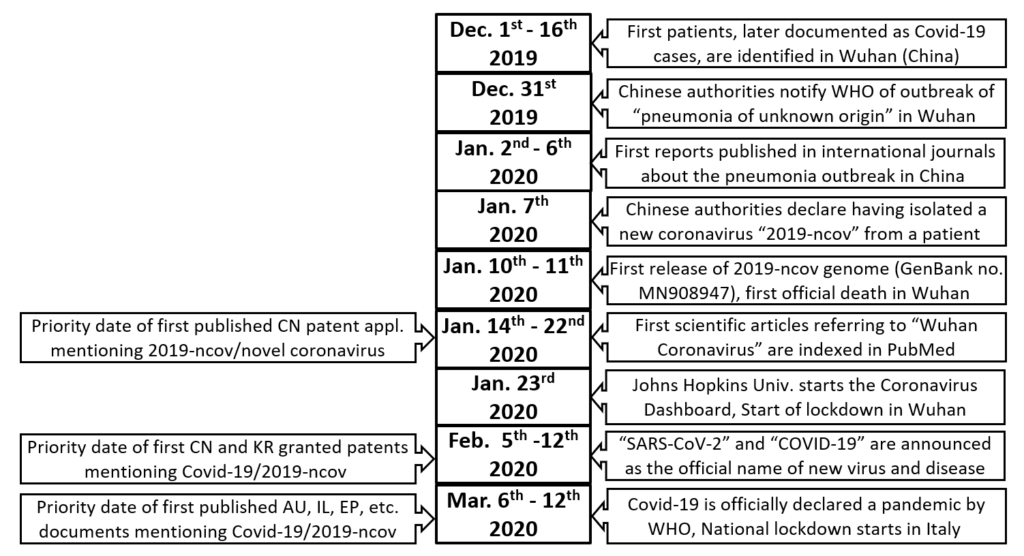
As somehow expected given the emergency situation and the timing, these early patent filings disclose very few examples of novel chemical or biological entities to be administered using pharmaceutical compositions, showing a prevalence of “repurposed” compounds for treating the disease or blocking the infection. More than 80% of the patent documents identified by the search have been filed at the Chinese or Indian Patent Office, many of them referring to traditional medicine products or to low-technology products but quite a few disclosing more sophisticated technologies such as diagnostic tools and vaccines (for instance, a Chinese patent has been granted for the vaccine developed by CanSinoBIO). Other jurisdictions where a substantial number of patent documents has been identified are the United Kingdom (but mostly notified only in the UK Patent Journal), Australia (mostly as provisional patent applications or Innovation Patents), South Korea (some already granted), USA (but mostly as Continuation-in-Part applications), Singapore (all provisional patent applications), Germany (but only as utility models), Russia, Israel, Brazil, and Spain. Almost no example of accelerated examination and publication for patent filings related to COVID-19 were found at WIPO, EPO, or JPO, at least in this very early phase.
This analysis of patent publications relating to COVID-19 will soon be updated with data available in the period September 2020 – February 2021 and later on during 2021. It will be interesting to see any effect of changes in the subsidies that Chinese authorities have allocated to local companies and institutions for pursuing patent filings in China in recent years, as reported in this recent official communication from Chinese authorities, a policy that is indicated as a major “non-market factor” impacting upon filing trends and IP systems in China in a dedicated report issued by USPTO in January 2021. Another topic to evaluate is how patent protection strategies are pursued in parallel to clinical and commercial activities related to COVID-19 vaccines, diagnostics, and therapeutics (for example, the authors of a recent Lancet paper about the Sputnik V vaccine are named as inventors in a series of Russian patent documents published between May and September 2020 and in a PCT application published in January 2021).
Almost certainly, a major issue for patentability analysis of claims referring to COVID-19 subject matter will be the prior art effect of hectic publication trends for articles and preprints relating to this pandemic. Just to give an idea of the turmoil in the scientific publications, the National Institute of Health (NIH) has established a dedicated literature database (LitCovid) indicating that at least 2,000 papers relating to COVID-19 have been published each week since May 2020. PubMed, the main biomedical literature database provided by NIH, indexes between 400 and 900 papers about general Coronavirus-related research each year for the 2010s decade, while more than 61,000 publications are indexed by PubMed for the same topic in 2020. This latter figure can be compared with publications that are indexed for 2020 either in the even more comprehensive Global Literature on Coronavirus Disease Database (provided by the World Health Organization and listing more than 180,000 publications) or still in PubMed for other health topics (for instance, more than 210,000 publications relating to cancer but less than 5,000 to malaria).
Indeed, it is also important to mention the consequences of policies that many scientific publishers imposed to authors and institutions in recent years about declaring any parallel patent filings as a potential conflict-of-interest. This obligation, even though not uniformly applied and respected in reality, may have the effect of disclosing inventorship, applicant’s name, and/or other filing details for patent applications within articles, but at a date when such patent applications are, in general, still to be published. The format and the wording that is applied by authors in this text is quite variable, but this content may be still quite informative, as shown in some examples taken from Journal of Clinical Investigation, Heliyon, Nature Communication, Proc Natl Acad Sci U S A, and Science.
Any commentary or proposal about this study can be forwarded to the authors.
________________________
To make sure you do not miss out on regular updates from the Kluwer Patent Blog, please subscribe here.



I’ve been reading about and researching the top Covid 19 Vaccines and I came across your website. I found your article very helpful and would like to thank you for it.Like many people out there, I am extremely worried about the negative publicity most vaccines have received.
I’d like to share with you this unique guide I came across, that provides unbiased information on the top 5 vaccines and a bit more.It really put me at ease and I now have a better understanding of what is available out there. You can find the guide here: https://www.dnaweekly.com/blog/covid-19-vaccine-ultimate-guide/
I am sure it will help your readers in the same way it helped me.