Preliminary injunctions are powerful weapons in patent infringement suits. A successful application – and sometimes even an unsuccessful application – for a preliminary injunction (“PI”) will often lead to an early resolution of litigation. Chinese law authorizes courts to issue PIs, either before or during an infringement action. As such, there are two kinds of PIs: pre-suit injunctions (sought before filing a main infringement case) and in-suit injunctions (sought during the pendency of a main infringement case). Early enthusiasm in granting PIs by courts was tempered by words of caution and restraint from the Chinese Supreme Court. China since then went through years of PI “darkness.” Recently, the pendulum has swung back – PIs have come back with a vengeance.
Procedures
Upon receiving a request for a pre-suit injunction, a court must make a ruling within 48 hours if it finds that all procedural requirements have been properly met. The 48-hour time limit can be extended for another 48 hours or longer in complex cases. Once issued, the injunction is immediately enforceable. The patentee, if it has not done so already, then must initiate an infringement action in the court within 30 days of issuance of the injunction, or the injunction automatically will be lifted. The issuance of a pre-suit injunction is not appealable; but the enjoined party may request the issuing court to reconsider its decision, which is an administrative procedure within the court. However, the injunction will remain enforceable during reconsideration and any subsequent infringement proceedings until final judgment. In-suit injunctions are sought during litigation and thus not subject to the 48-hour time limit. Similar to a pre-suit injunction, the grant of an in-suit inunction is also not appealable but subject to reconsideration. It is also enforceable until final judgment.
Substantive Factors
Chinese courts generally consider the following factors in determining whether to issue PIs in patent cases:
(a) whether there is patent infringement;
(b) whether the patent holder will be irreparably harmed in a manner for which monetary damages are inadequate compensation if the infringing act is not enjoined;
(c) whether the patent holder has provided an adequate bond; and
(d) whether issuance of a PI would prejudice the public interest.
Practical Difficulty
In practice, however, obtaining a PI in patent infringement cases has seen its fair share of difficulty. Both infringement and irreparable harm must be clearly proven – a burden that is not easy to meet in China, given the stringent evidentiary requirements and lack of discovery procedures. In early 2000, Chinese courts experimented with some relatively aggressive PIs in patent cases. Unfortunately, some of the PIs were wrongly issued. For example, the patent in suit in some cases was later invalidated. That prompted courts to be less enthusiastic. By mid-2000, the Chinese Supreme Court started to urge caution in issuing PIs and instructed lower courts that PIs should not be issued in patent cases involving non-literal infringement or complicated technologies. The judicial conservatism heightened through the rest of 2000, with few PIs issued in patent cases in China.
PI Statistics
Generally speaking, most IP suits in China do not involve an application for a PI. The following statistics are a snapshot for 2010-13.
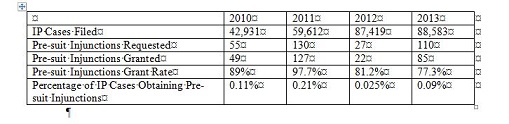
While the grant rate is rather high among those who sought a pre-suit injunction, the overwhelming majority of the IP cases do not get the benefit of pre-suit injunctions. Only clear cases of infringement are likely to get a pre-suit injunction, assuming other requirements, such as irreparable harm and adequate bond, are met. There are no statistics for in-suit injunctions; but it is safe to assume that they are similar to pre-suit injunctions.
Changing Tides
In mid-2013, the pendulum started to swing back, with the Chinese Supreme Court urging a balance of proactivity and reasonableness in granting PIs. This set the stage for some landmark PI rulings.
A. First Patent PI in Beijing
The Beijing courts had never issued a PI based on patent infringement until Abbott Laboratories sought to enforce its design patent on its Similac® SimplePac design before the Beijing Third Intermediate Court in November 2013. The accused infringing product was almost identical to Abbott’s patented design (shown below).
The Beijing Third Intermediate Court found that (1) there was a high likelihood that Abbott could prevail on its infringement claim; (2) Abbott would suffer irreparable harm; (3) Abbott’s harm, if a PI was not issued, would be greater than the infringers’ harm if a PI was issued; (4) Abbott posted an adequate bond; and (5) no public interest would be adversely impacted. Accordingly, the court issued a pre-suit injunction, prohibiting the infringing party from making, selling, or offering to sell the infringing products. This is the first PI based on patent infringement in the history of the Beijing courts.
B. First PI against Cross-Label Use in China
About three months after the first PI obtained by Abbott, the Beijing Second Intermediate Court granted Novartis’ request for a PI against a generic company for infringing its indication patent relating to the drug Glivec®. The active pharmaceutical ingredient for the Glivec® drug is imatinib mesylate. While the compound patent on imatinib mesylate has expired, the use of imatinib mesylate for the treatment of gastrointestinal stromal tumor, a form of stomach cancer, is still protected by the indication patent. However, the generic company’s drug insert and promotional materials for the generic imatinib mesylate contained certain information regarding the use of this drug for the treatment of gastrointestinal stromal tumor. Finding for Novartis, the Beijing Second Intermediate Court issued an in-suit injunction, prohibiting the defendants from making any reference in their drug insert to “the treatment of gastrointestinal stromal tumor” in the course of the manufacture, sales, and offer for sales of the generic imatinib. It is noteworthy that this is the first PI against cross-label use in China.
C. PIs in Trade Secrets Cases
PIs in patent cases are not the only ones that are getting traction in China. In January 2014, the Shanghai First Intermediate Court issued China’s first pre-suit injunction in a trade secret dispute between a Chinese subsidiary of Novartis and its former employee. This is the same court that issued China’s first in-suit injunction in a trade secrets case in July 2013, where the PI was applied for after Eli Lilly had brought a trade secret claim against its ex-employee. In the Novartis case, the ex-employee downloaded about 880 documents containing trade secrets from the company’s database after he resigned. He later joined a competing company. Novartis timely applied for a pre-suit injunction against the ex-employee before the Shanghai No. 1 Intermediate People’s Court, seeking to restrain the ex-employee from disclosing, using, or allowing others to use the documents containing trade secrets and related confidential information. The court accepted the petition on the same day and issued a PI order within 48 hours thereafter.
Concluding Thoughts
The recent groundbreaking PIs issued by the Beijing and Shanghai courts are exciting developments. They should motivate others to follow suit. Does it mean that it is easier to get a PI in China now? The answer appears to be yes. PIs in trade secrets cases were not possible before 2013. In the meantime, PIs in patent cases were on their death bed until mid-2013 when the Chinese Supreme Court retreated from its past conservatism and started to urge proactivity and reasonableness in PI adjudication. Notwithstanding the new glimmer of hope, it won’t be easy to get patent based PIs, which are likely to be an exclusive province for clear cases of infringement (like everywhere else in the world).
________________________
To make sure you do not miss out on regular updates from the Kluwer Patent Blog, please subscribe here.