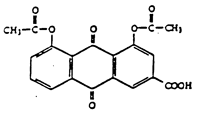
Laboratoires Negma (hereinafter referred to as “Negma”) is the exclusive licensee of European patent No. 0 520 414 which relates to a method for the preparation of diacetylrhein (also called diacerein) having a specific degree of purity as well as diacetylrhein obtained by this process and a pharmaceutical composition containing this compound.
Such European patent was first filed and owned by Madaus AG and then assigned to Laboratoire Medidom (hereinafter referred to as “Medidom”).
Negma first sent a cease and desist letter to Biogaran (a generic drug company) claiming that Biogaran’s diacerein products, for which it obtained three marketing authorisations, infringed European patent No. 0 520 414 under which it has an exclusive licence.
As a response and preventive action, on 12 December 2008, Biogaran summoned Medidom and Negma before the Tribunal de Grande Instance of Paris for the revocation of claim 14 of the said patent for lack of novelty and inventive step. And Biogaran began marketing its own products.
Therefore, less than two months later, Negma sought a preliminary injunction against Biogaran before the Tribunal de Grande Instance of Strasbourg, which was granted on 10 March 2009 (injunction, under penalty from marketing, arranging to distribute, manufacturing or arranging to manufacture the generic pharmaceutical products and to recall these products under penalty as well).
Biogaran then obtained from the Tribunal de Grande Instance of Paris the revocation of claim 14 of European patent No. 0 520 414, in a judgment dated 31 March 2010 which was later upheld by the Cour d’Appel of Paris on 30 June 2010, ruling in fast-track proceedings.
Taking into account the 31 March 2010 judgment of the Tribunal de Grande Instance of Paris which had revoked claim 14 of European patent No. 0 520 414, the Cour d’Appel of Colmar reversed, on 22 June 2010, the preliminary injunction granted against Biogaran by the Tribunal de Grande Instance of Strasbourg on 10 March 2009 .
As a consequence, Biogaran sought, before the Tribunal de Grande Instance of Paris, compensation for the damage suffered due to the injunction granted at Negma’s and Medidom’s benefit.
In a 27 January 2012 decision, the Tribunal de Grande Instance of Paris holds the following:
- it dismisses all the claims against Medidom (the patent owner) stating that it was not party to the proceedings from which resulted the preliminary injunction;
- it dismisses Biogaran’s claims based on a tort liability (i.e. on the basis of Article 1382 of the French Civil Code) because of the lack of evidence of distinct faults, and notably:
- Negma did not use a blocking strategy before the AFSSAPS (the French agency for the safety of health products, which grants, suspends or withdraws marketing authorisations for medicines) during the proceedings to obtain marketing authorisations but only developed arguments that the AFSSAPS, as an independent entity, was free to take into account or not;
- Negma’s attempts for challenging the decision of the Comité Économique des Produits de Santé (CEPS i.e. the French economic committee for health products) cannot constitute a dilatory manoeuvre since Negma only used its right to challenge an administrative decision;
- Negma did not misrepresent its capacity to act as an exclusive licensee to obtain a preliminary injunction;
- Negma’s letters to pharmacists and wholesalers reproducing part of a decision obtained before the Conseil d’Etat (i.e. the French highest administrative court) did not distort such decision;
- Negma did not abuse its rights by bringing proceedings based on its patent which was, at the time the measures were requested, valid;
- There was no evidence that Negma committed anti-competing practices to prevent Biogaran from penetrating the market; none of the conditions for anti-competing practices (such as dominant position and abuse) were fulfilled.
- Biogaran’s loss of contribution margin resulting from the preliminary injunction to stop marketing the litigious products;
- Biogaran’s costs due to the recall of its products from the market;
- Biogaran’s costs for the retreatment of its products in stock required for putting them on the market again.
On the other hand, the court holds Negma liable on the basis of Article 31 of the French Act of 9 July 1991 and orders Negma to compensate Biogaran for the profit it would have made should the injunction to stop selling the generic pharmaceuticals products have not been enforced.
Such liability is based on Article 31 of the French Act of 9 July 1991 which provides: “The enforcement on the basis of a provisionally enforceable title may be carried out until it is completed. The enforcement is carried out at the risk of the creditor, who shall restore the debtor’s rights in kind or by an equivalent, should the title be subsequently modified”.
Firstly, the Tribunal de Grande Instance of Paris refuses to set aside this Article 31 whose provisions are compatible with Article 9.7 of Directive 2004/48/EC on the enforcement of intellectual property rights, with Article 50.7 of the TRIPS Agreement and even with Article 6§1 of the European Convention for the Protection of Human Rights (which refers to the right to a fair trial).
It is interesting to note that in the same proceedings, the judge in charge of the case preparation of the Tribunal de Grande Instance of Paris had to rule on a priority question of constitutionality (Question prioritaire de constitutionnalité) raised by Negma and claiming that Article 31 of the French Act of 9 July 1991 violated the constitutional property right.
The judge in charge of the case preparation decided, on 21 October 2011, that such question was not serious and refused to refer it to the Cour de Cassation (the French highest judiciary court, which is the first step before the referral of a priority question of constitutionality to the Conseil Constitutionnel, the French constitutional court). This decision interestingly recalls that the provisions of the 1991 Act organise a fair balance between the claimant who takes the risk of enforcing a provisional decision and the defendant who is given the possibility to obtain damages if the enforced decision is later reversed.
Secondly, the Tribunal de Grande Instance of Paris dismisses Negma’s argument according to which the liability resulting from this article shall depend on the demonstration of a fault. The provisions of Article 31 of the French Act of 9 July 1991 are clear and do not need any interpretation whatsoever. This article provides a liability for the harmful consequences of an enforcement of a court decision without needing to demonstrate a fault. And it applies whether the decision was enforced upon or voluntarily complied with by the debtor.
In this particular case, it was not disputed that Negma had clearly shown its intention of enforcing the decision since it had served the order dated 10 March 2009 on Biogaran on 12 March 2009, sent a letter to Biogaran to ensure the provisional enforcement of the order and started proceedings for the calculation of the penalty set in the said order.
And, consequently, the Tribunal de Grande Instance of Paris sets Biogaran’s damages at €2,997,567 (when Biogaran claimed €8,282,213) including:
It should be noted that the court reduced the amount of the damages claimed by Biogaran on the ground that it would have not remained the only generic drug company on the market during the time its product was removed from the market.
Indeed, at least five generic drug companies were ready to launch their product but were only waiting for Medidom’s patent (or at least its claim 14) to be revoked.
Furthermore, it seems that the court granted part of Biogaran’s lost margin resulting from the preliminary injunction for the period following the reversal of this order by the Cour d’Appel of Colmar on 22 June 2010.
This period could correspond to the time needed to get back to a normal economic situation, after the injunction (which was claimed by Biogaran).
The judgment handed down on 27 January 2012 is the clear recognition of the following principle: enforcing a preliminary injunction, before the patent claim on the basis of which the injunction had been granted is declared invalid on the merits, leads to a liability for the harmful consequences of this injunction without needing to demonstrate a fault.
The above cited principle may be expanded to any and all enforcements of a court decision, and not limited to preliminary injunctions.
Original French decision.
English translation .
Author: Marta Mendes Moreira, Attorney-at-law, Véron & Associés, Paris, France
________________________
To make sure you do not miss out on regular updates from the Kluwer Patent Blog, please subscribe here.


