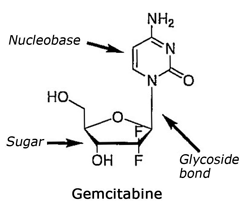
Gemcitabine is part of the family of nucleosides which are compounds constituted of two chemical parts: a sugar bound to a nucleobase through a glycosidic bond. As any nucleoside can have two isomers (the alpha-anomer and the beta-anomer) depending on the orientation in space of the glycosidic bond, it must be clarified that Gemcitabine is a nucleoside of the beta-anomer type where the nucleobase is situated above the sugar part. This beta-anomer constitutes Gemcitabine and presents a therapeutic effect whereas the alpha-anomer, where the nucleobase is situated below the sugar part, has no therapeutic effect.
Gemcitabine is used as an antiviral and antineoplastic medicine and marketed in France by the U.S. company Eli Lilly (hereinafter referred to as Eli Lilly) under the trade name Gemzar.
Eli Lilly has already been the holder of the basic patent EP 122 707, covering Gemcitabine, which has been the subject-matter, in France, of an SPC expired in March 2009.
But Eli Lilly is also the holder of European patent EP 0 577 303 whose subject matter is a “stereoselective glycosylation process” for preparing 2’-deoxy-2,2-difluoro-beta-nucleosides, which include Gemcitabine.
The subject-matter of the patent is a synthesis process of an beta-anomer (Gemcitabine) enriched mixture consisting in using an alpha-anomer enriched starting carbohydrate, including a mesilate leaving group, which reacts with another compound, a nucleobase, through a glycosylation reaction performed by means of a second-order nucleophilic substitution (SN2; a nucleophilic substitution may only be of two types, SN1, first-order nucleophilic substitution, or SN2, second-order nucleophilic substitution), and in deblocking to achieve a mixture containing more beta-anomer (Gemcitabine) than alpha-anomer by the effect of an anomeric inversion.
That is the reason why the patented process is a “stereoselective glycosylation process”: it fundamentally consists in a glycosylation reaction aiming at a high yield of beta-anomer, at producing preferentially a certain type of anomer, an alpha-to-beta ratio greater than 1:1. This idea of an improved yield does not appear in claim 1 but in the description so that the Cour d’Appel of Paris is of the opinion that any product in which the beta-to-alpha anomeric ratio is greater than 1:1 falls within the scope of claim 1.
The company Sandoz France (hereinafter referred to as Sandoz) produces and places on the French market, as holder of the marketing authorisation, generic substitutes of the Eli Lilly products.
Considering that, using the new invention covered by patent EP 0 577 303 as pretext, Eli Lilly retained its monopoly of use arising from the patent covering the Gemcitabine product fallen in the public domain, Sandoz summoned Eli Lilly before the Tribunal de Grande Instance of Paris for the revocation of the French designation of European patent for lack of inventive step.
The Tribunal de Grande Instance of Paris having dismissed, on 2 July 2010, Sandoz’s claim for invalidity of the claims of the European patent EP 0 577 303, the latter lodged an appeal before the Cour d’Appel of Paris, maintaining again the lack of inventive step but maintaining also the invalidity of the patent for insufficient disclosure.
In a 13 January 2012 decision, the Cour d’Appel of Paris affirms the judgment handed down by the Tribunal de Grande Instance of Paris, decides that the patent claims involved an inventive step and dismisses the claim for invalidity of the patent for insufficient disclosure.
The decision is very interesting because of its very detailed appraisal of the inventive step.
From a pure legal point of view, it firstly recalls – and constitutes so an example in this respect – that, in contrast to the assessment of novelty which shall only be based on a comparison of the claimed invention with each individual item from the prior art (see EPO, Case Law of the Boards of Appeal of the European Patent Office, 6th ed. 2010, p. 109), the assessment of inventive step may be based on each item or a combination of items from the prior art. In the present decision, inventive step has been assessed over each Hertel, Chou and Howell document but also over the Chou and Howell combination.
It may be underlined also that the Cour d’Appel has applied the “problem and solution approach”, recommended and applied within the EPO, to assess inventive step. The formal divisions of the decision even reflect the successive steps of the problem and solution approach as defined by the case law of the Boards of Appeal of the European Patent Office.
So, this problem and solution approach (see EPO, Case Law of the Boards of Appeal of the European Patent Office, 6th ed. 2010, p. 162) consists essentially of:
a) identifying the “closest prior art”, (“4 – The state of the art at the priority date of the patent” which states that the litigants agree that the publications disseminated by Mr. Hertel and Mr. Chou, relating to the production of Gemcitabine, were the closest prior art at the priority date of the patent in question and that Sandoz also adds the Howell document, relating to the synthesis of a type of nucleosides other than Gemcitabine).
b) assessing the technical results (or effects) achieved by the claimed invention when compared with the “closest state of the art” established, (“4 – 1 The Chou and Hertel documents”; “4 – 2 The Howell document”)
c) defining the technical problem to be solved as the object of the invention to achieve these results, and (“5 – On the technical problem to be solved”)
d) examining whether or not a skilled person, having regard to the closest state of the art, would have suggested the claimed technical features in order to obtain the results achieved by the claimed invention (“6 – On the inventive step of claim 1”; “6 – 1 Inventive step over the Hertel document? ”; “6 – 2 Inventive step over the Chou document?”; “6 – 3 Inventive step over the Howell document?”; “6 – 4 Inventive step over the Chou and Howell combination? ”).
About this last step of the problem and solution approach, the Cour d’Appel also draws from the case law of the Boards of Appeal of the European Patent Office, the idea that “the point is not whether the skilled person could have arrived at the invention by modifying the prior art, but rather whether, in expectation of the advantages actually achieved (i.e. in the light of the technical problem addressed), he would have done so because of promptings in the prior art” (EPO, Case Law of the Boards of Appeal of the European Patent Office, 6th ed. 2010, p. 177). The person skilled in the art, in the present case, should be a specialist in organic chemistry, more particularly in the field of sugar chemistry, and still more particularly in that of the stereoselective synthesis of nucleosides. He must possess strong basic general kwowledge in these specialities without however being a researcher who devotes his activities to cutting-edge research.
From theory to the practice of the present case:
1) firstly, the Cour d’Appel confirms that the person skilled in the art would not have found, without himself engaging in any inventive step, in the Hertel document any incentive to achieve the subject-matter of claim 1. The court argues that the Hertel document differed from the claimed invention in the starting product which is a sugar mesilate in an alpha-to-beta anomeric ratio of 1:1, the protecting groups of the sugar, the nucleophilic substitution mechanism of the SN1 type, the final alpha-to-beta anomeric ratio of 4:1 (and then not greater than 1:1) and that the person skilled in the art, who was aware of the Hertel document, would have first sought to improve the synthesis process of Gemcitabine beginning by modifying the protecting groups of sugar, which was done in the Chou document with only an anomeric ratio of 1:1, as a result.
2) the Cour d’Appel then confirms that the person skilled in the art would not have found, without himself engaging in any inventive step, in the Chou document any incentive to achieve the subject-matter of claim 1. The Chou document described the production of a 2’-deoxy-2,2-difluoronucleoside through a nucleophilic substitution reaction using a leaving group sulfonyloxy such as mesilate, through a process which led to an alpha-to-beta anomeric ratio of 1:1. The Chou document differed from the claimed invention in its starting product which was not alpha-anomer enriched by a stereoselective process, the nucleophilic substitution mechanism (SN1) and the final product (a mixture with an alpha-to-beta anomeric ratio of 1:1). To achieve the invention from this document, the person skilled in the art should first enrich the starting sugar mesilate to distinguish it from the alpha/beta mixture in a 1:1 ratio, then perform glycosylation by the SN2 path to obtain a beta-anomer enriched nucleoside of the Gemcitabine type. However, the person skilled in the art did not have at his disposal, at the priority date, a stereoselective synthesis process to obtain an alpha-anomer enriched starting product – a sugar mesilate – the processes used before to achieve it being long, complex and costly. Moreover, Eli Lilly through the stereoselective process leading to an enrichment in starting carbohydrates in the form of alpha-anomer, which it had patented on the same day than the process in question, had opened up the way to an SN2. Since the Chou document only taught the SN1 path, the person skilled in the art would not have been encouraged (neither by the teaching of the Chou document, nor by the alpha-anomer enrichment processes at his disposal at the priority date) to exploit the SN2 path. The person skilled in the art would not have been encouraged to use a mesilate starting group to obtain Gemcitabine by the SN2 path or, if he had wanted to choose this path, he would have selected a leaving group other than mesilate, a halogenate group, bromine or chlorine for example.
3) the Cour d’Appel then also confirms that the person skilled in the art would not have found, without himself engaging in any inventive step, in the Howell document any incentive to achieve the subject-matter of claim 1. The Howell document effectively sought the preferential formation of beta-anomer through an SN2 like in the patent. However, besides the fact that the Howell process did not apply to the synthesis of Gemcitabine, the alpha-anomer enriched starting carbohydrate showed two differences: the carbohydrate in the Howell document has a single fluorine atom at the C-2 position while, in the patent, it has two fluorine atoms at that position; the bromine atom constitutes the leaving group in the prior art document while a sulfonyloxy or sulfonate or mesilate is part of the starting product in the claimed invention. And the person skilled in the art knew that the sulfonyloxy groups, including mesilate, were excellent leaving groups, better than halogens such as bromine or chlorine, and he also knew that their use led to an SN1 reaction, which did not encourage him to use them since he knew that they did not make it possible to obtain an SN2 reaction.
4) the Cour d’Appel finally confirms that the person skilled in the art would not have found, without himself engaging in any inventive step, in the Chou and Howell combination any incentive to achieve the subject-matter of claim 1. According to Sandoz, in order to obtain an excess of beta-anomer, the person skilled in the art would have been encouraged to apply the SN2 reaction described in the Howell document to the Chou starting products (mesilate) to achieve the claimed invention. The Cour d’Appel does not approve this allegation. It considers that the mesilate leaving group of the Chou document is not suited to solving the technical problem of the patent, which is to obtain Gemcitabine in an anomeric ratio greater than 1:1, the person skilled in the art would have then contemplated taking the halogen leaving group taught by the Howell document, but would not have achieved the subject-matter of the claim since he would have missed the first step, which is the stereoselective production of the (alpha-anomer enriched) sugar mesilate as the starting product. It also considers that Professor Beau’s assertions, underlined by Sandoz, rest on the mere supposition that the person skilled in the art could have used the information acquired on the monofluorated derivatives (Howell) to modify the conditions of the glycosylation reaction in the desired direction, but do not contain sufficient indications to explain why he would have been encouraged to implement them or if he would have done so in the hopes of finding a solution to the posed technical problem, which is to obtain a beta-anomer enriched nucleoside by using the SN2 path from an alpha-anomer enriched carbohydrate of a defined formula.
Claim 1 involving an inventive step, claims 2 to 14, all directly or indirectly dependent of claim 1, should also be considered as involving an inventive step.
Finally, the decision of the Cour d’Appel is also interesting on the insufficiency of disclosure which Sandoz has claimed for the very first time in appeal. The court also dismisses this claim because of the indications and examples given by the description (“Consequently, the description contains sufficient information to enable the person skilled in the art to carry out the invention”) but also because of the failure of Sandoz, on which was the onus of proof, to provide “beyond a reasonable doubt” the proof of the insufficiency of disclosure. The court introduces this standard (“beyond a reasonable doubt”) which is not familiar to French evidence law and rather seems directly taken from the case law of the Boards of Appeal of the European Patent Office where it is a familiar standard of proof (see EPO, Case Law of the Boards of Appeal of the European Patent Office, 6th ed. 2010, p. 73, 87-91, 290, 306, 373, 429, 499, 558-561, 564, 602, 651, 677, 942). This requirement of a proof “beyond a reasonable doubt” is nothing less than the requirement of a “degree of high certainty” “close to absolute conviction”.
And in the present case, the Cour d’Appel notes: “If each party is responsible for proving, in accordance with the law, the facts necessary to the success of its claim, the party which maintains that the invention is not sufficiently disclosed should provide proof, on the balance of probabilities, that the person skilled in the art would be unable to carry out the invention based only on his scientific and technological knowledge, it being specified that proof should be provided beyond a reasonable doubt and that the benefit of the doubt should be given to the patent holder”.
Original French decision.
English translation .
Author: Nicolas Bouche, Head Legal Research and Literature, Véron & Associés, Paris, France
________________________
To make sure you do not miss out on regular updates from the Kluwer Patent Blog, please subscribe here.


