H. Lundbeck A/S (hereinafter referred to as Lundbeck) is the holder of European patent EP 0 347 066 entitled “new enantiomers and their isolation”, which designates France and was filed on 1 June 1989; it claims priority of a British patent dated 14 June 1988.
The invention relates to the two new enantiomers of the antidepressant drug Citalopram and the use of these enantiomers as antidepressant compounds as well as their possible use in geriatrics or in the treatment of obesity and alcoholism.
The patentee explains that the known compound, Citalopram, which was disclosed, for example, in US patent No. 4,136,193 has proven to be an efficient compound in man and that the work in the development of this compound was made with the racemate. He adds that Citalopram has been shown pharmaceutically to be a very selective inhibitor of 5-HT (or serotonin) reuptake but that previous attempts to crystallize diastereomeric salts of Citalopram enantiomers have failed.
He then mentions that surprisingly, it has proven possible to resolve the intermediate product II (diol) into its enantiomers and, finally, in a stereoselective way, to convert these enantiomers into the corresponding Citalopram enantiomers.
He mentions that (page 3 of the description, lines 15 to 17) “furthermore, it was shown to our surprise that almost the entire 5-HT uptake inhibition resided in the (+)-citalopram enantiomer”.
The protection of this patent in France was extended by means of the corresponding supplementary protection certificate (SPC) No. 02 C 0050. And, on the basis of this patent, Lundbeck markets an antidepressant drug called SEROPLEX (INN – Escitalopram) for which it was granted a marketing authorisation (MA) in the European Community on 7 December 2001.
By way of an act dated 8 March 2007, Ratiopharm GmbH (hereinafter referred to as Ratiopharm) served a summons upon Lundbeck before the Tribunal de Grande Instance of Paris for the invalidity of claims 1 to 5 of the French designation of European patent EP 0 347 066, for lack of novelty or of inventive step and for the invalidity of the corresponding SPC No. 02 C 0050.
The five claims at issue read as follows:
1 – (+)–1–(3-dimethylaminopropy1)–1–(4′-fluoropheny1)–1, 3–dihydroisobenzofuran–5–carbonitrile of the following general formula
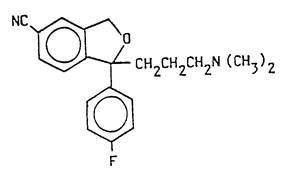
and nontoxic acid addition salts thereof.
2 – The pamoic acid addition salt of the compound in claim 1.
3 – A pharmaceutical composition in unit dosage form comprising, as an active ingredient, a compound as defined in claim 1.
4 – A pharmaceutical composition in unit dosage form comprising, as an active ingredient, the compound of claim 2.
5 – A pharmaceutical composition in unit dosage form, according to claim 3 or 4, wherein the active ingredient is present in an amount from 0.1 to 100 milligram per unit dose.
In a 30 September 2010 decision, the Tribunal de Grande Instance of Paris held admissible Ratiopharm’s action for invalidity but held valid both claims 1 to 5 of the French designation of European patent EP 0 347 066 and, in consequence, the corresponding supplementary protection certificate No. 02 C 0050.
On the admissibility of Ratiopharm’s action for invalidity:
Lundbeck asserted that Ratiopharm had based the admissibility of its action for invalidity on its marketing authorisation applications for generic drugs of the reference drug Seroplex. Since these applications in the meanwhile had been withdrawn in France or suspended in the other European countries, Lundbeck concluded that Ratiopharm’s action was therefore inadmissible for lack of interest. Ratiopharm could no longer be considered as a direct or potential competitor because it cannot prove that it meets the administrative rules that subject any marketing to the grant of an MA.
However, the Tribunal rejected this claim firstly because it is settled case law that the interest in taking an action is appraised on the day when legal proceedings are instituted. This has been expressed in general terms by the French Cour de Cassation (see Cass. civ. 2nd, 13 February 2003, No. 01-03272, Bull. civ. II No. 34; D. 2004. IR. 805) and by the Cour d’Appel of Paris especially in the context of an action for the invalidity of a patent (Paris, 4th Ch., 26 May 2000, Ann. propr. ind. 2/2000, p. 184). And, secondly, since no text specifies the persons who are authorised to lodge an action for patent invalidity and, in particular, for a patent relating to a medicinal product, the general procedure principle is that the right of action is available to all those who have a legitimate interest in the success or dismissal of a claim, pursuant to the wording of Article 31 of the French Code of Civil Procedure. And, according to the Tribunal, that interested person, i.e. having an interest in taking an action for patent invalidity, is “every current or potential competitor” of the patentee.
In the present case, at the date when the summons was served, namely on 8 March 2007, Ratiopharm’s will to market generic drugs of the reference drug Seroplex was demonstrated by the MA applications in France pending before the AFSSAPS and by the MAs already granted in other European countries. Moreover, Ratiopharm is marketing a generic drug of Citalopram whose therapeutic indications are very close to Escitalopram. So that the existence of the patent at issue and of the SPC relating to it could hinder the development of its activity in the field of antidepressant drugs belonging to the family of serotonin reuptake inhibitors. And the fact that Ratiopharm’s MA applications have been withdrawn or suspended afterwards was irrelevant.
On the validity of the French designation of European patent EP 0 347 066:
– firstly, the Tribunal rejects the claim for lack of novelty:
Ratiopharm relied on two prior art documents namely French patent No. 2 338 271 filed on 14 January 1977 by Kefalas, which described Citalopram, and European patent No. 0 171 943 filed on 19 July 1985 by Lundbeck, which described an intermediate for the synthesis of Citalopram and a route for the synthesis of Citalopram. According to Ratiopharm, these patents by describing Citalopram which is a racemate, i.e. a mixture of the two enantiomers in equal amounts, necessarily disclosed the (+)enantiomer, the subject-matter of claim 1 of the patent at issue, and described the means for the isolation thereof and for testing its antidepressant activity in order to verify some technical knowledge that still identifies with claim 1 of patent at issue.
The Tribunal underlines the fact that if the claimed prior art documents described Citalopram, a racemic compound comprising the (+)enantiomer, they, however, did not teach to the skilled person, at the priority date of the allegedly invalid patent, the existence of this enantiomer as such and above all the possibility to isolate it. Then the invention subject-matter of claim 1, i.e. the Citalopram (+)enantiomer, could not be found by the skilled person “in its entirety” in the prior art documents, “with the same elements composing it in the same form, the same arrangement and the same functioning with a view to achieve the same technical result”.
– secondly, the Tribunal rejects the claim for lack of inventive step: to this end, the Tribunal relies on the said “problem-solution approach” which is commonly applied by the EPO.
It first infers from the patent that the problem faced by the skilled person was to suggest an alternative to the known compound, Citalopram, which has turned out to be an efficient antidepressant compound for man.
It also infers therefrom that the skilled person must be defined as a team composed of a medicinal chemist, a pharmacologist and a biochemist, all being clinicians, working in the pharmaceutical industry. However, it refuses to add to this team an analytic chemist specialising in the analysis and the separation of organic molecules for therapeutic purposes because no element submitted to the discussion demonstrated that the problem raised was precisely the separation of the enantiomers composing Citalopram, unless one conducts a reasoning backwards, starting from the solution provided by the invention. By doing so (for another example of the skilled person understood as a team, see Cour d’Appel of Paris, Division 5, Chamber 1, 27 October 2010, Docket No. 09/08135, Johnson & Johnson, Ethicon v. Novartis) the Tribunal is clearly in opposition to the French Cour de Cassation which seemed to refuse such a composite conception of the skilled person in a 17 October 1995 decision (Cass. com., 17 October 1995, Ann. propr. ind. 1996, No. 1, p. 5, to assess an invention from Bosch, a striking and/or drilling tool, the Cour d’Appel wanted to use two different specialists: a tool specialist and a specialist in a specific type of machine; which the Cour de Cassation had refused).
The Tribunal certainly admits that the skilled person could be prompted to separate the enantiomers composing the chiral therapeutic molecule such as Citalopram, and in particular by the example of the first separation of enantiomers achieved at the end of 19th century by Louis Pasteur, by the US or Japanese administrative regulations and by the marketing of several chiral drugs in the form of one of the two enantiomers. However, the court holds that the skilled person, in the present case, would not have been prompted, because of the state of the art, to choose the separation of the Citalopram enantiomers to solve the problem he was faced with. As a matter of fact, Citalopram, as a racemic compound, was a satisfying molecule in the treatment of depression and presented no toxicity which would have prompted to separate the enantiomers in order not to administer a non-active compound that could turn out to be toxic. Moreover, the skilled person was not prompted to separate the enantiomers by the regulations or recommendations of the national authorities, this operation being not very easy but uncertain and expensive. Finally, the high performance liquid chromatography method (HPLC), taught by the patent, was not obvious to the skilled person at the priority date of the patent because it was still at an experimental stage in 1988 and the CHIRALCEL OD column necessary for its implementation was launched on the market in 1989 only.
Ratiopharm also disputed the inventive step of Lundbeck claiming that it was possible for the skilled person at the priority date of the patent to obtain Citalopram enantiomers using other enantiomers separation methods. The court first answers that although the skilled person knew well the method using the crystallization of diastereomeric salts of Citalopram, he would rapidly have rejected it because it emerged from the very description of the patent in dispute that the previous attempts had failed and that this method was known for the difficulties it caused and the uncertain results it provided. Concerning the stereospecific synthesis of the desired enantiomer, the court underlines again that it was not obvious, unless one conducts a reasoning backwards, starting from the solution provided by the invention, that the skilled person would have chosen at all costs to separate the Citalopram enantiomers to solve the problem he was faced with, in the absence of a prior art document that could justify such a choice.
Thus, the court held valid claims 1 to 5 of the French designation of European patent EP 0 347 066 held by Lundbeck.
On the validity of SPC No. 02 C 0050:
Ratiopharm asserted that the SPC was invalid pursuant to the provisions of Article 15 1° a) and Article 3 b), c) and d) of EC Regulation No. 1768/92, now No. 469/2009. Ratiopharm started again from the idea that Citalopram, as a racemic compound, mixing in equal amounts the (+)enantiomer, the only active principle, and the (-)enantiomer, which only has little effect and must be considered as an impurity or an inert compound. Consequently, MA NL 16 222 granted for Citalopram already constituted an MA granted for the (+)enantiomer, Escitalopram (and MA NL 27 538 mentioned in the application for the grant of SPC No. 02 C 0050 was not the first marketing authorisation for the product), and SPC No. 95 C 0009 granted for the KEFALAS Patent FR 2.338.271, which disclosed Citalopram, already constituted a SPC granted for the (+)enantiomer (and SPC No. 02 C 0050 was not the first SPC granted for the same product).
The Tribunal refuses again to agree with Ratiopharm. It firstly refuses to consider as an impurity the (-)enantiomer which, regardless of its activity, is a substance that contributes to the pharmaceutical activity of the Citalopram racemate. Indeed, for a common therapeutic indication, the Escitalopram dosage (20 mg) does not correspond to half the Citalopram dosage (60 mg). And the court then holds that the isolated enantiomer is an active principle distinct from the racemate. The subject-matter of MA NL 16 222 is a drug having the racemic form of Citalopram and not a mixture of its enantiomers whereas the subject-matter of MA NL 27 538 is a drug having the form of an enantiomer, Escitalopram. The product subject of the SPC at issue is another product than Citalopram as the racemic form and an individual enantiomer are distinct active principles presenting specific and distinct mechanisms of pharmacologic action. It finally underlines that Escitalopram which is the subject-matter of claim 1 of European patent EP 0 347 066 cannot be considered as a simple Citalopram derivative, as it is a new product pursuant to patent law.
Original French decision.
English translation.
Author: Nicolas Bouche, Head Legal Research and Literature, Véron & Associés, Paris, France
________________________
To make sure you do not miss out on regular updates from the Kluwer Patent Blog, please subscribe here.



One comment
Comments are closed.