The company Laboratoires Medidom (hereinafter referred to as “Medidom”) is the holder of European patent No. 520 414, entitled “Process for the preparation of diacetylrhein”, which concerns “a process for the preparation of diacetylrhein having a degree of purity making it suitable for use in pharmacies and having a total residual content of undesirable aloe-emodin of less than 20 ppm, as well as diacetylrhein that may be obtained by this procedure and a pharmaceutical composition containing this compound”. The company Laboratoires Negma (hereinafter referred to as “Negma”), the exclusively licence-holder of this European patent, commercialises it on the French market in the form of the drug ART 50 recommended in treating arthritic illnesses.
Negma, considering that the company Biogaran (hereinafter referred to as “Biogaran”) was about to put on the market two drugs which, being generic drugs of ART 50, were infringing European patent No. 520 414, sent to Biogaran a letter of warning.
As a response, Biogaran served a summons upon Medidom and Negma before the Tribunal de Grande Instance of Paris for invalidity of patent claim 14.
Since on 31 March 2010 the Tribunal de Grande Instance of Paris (3rd ch., 3rd sect., Docket No. 08/17625) had held claim 14 of the French designation of the European patent to be invalid for lack of novelty, Negma and Medidom then lodged an appeal against that decision.
However, in its 30 June 2010 decision, the Cour d’Appel of Paris (Division 5, ch. 1, Docket No. 10/07477) upheld the decision of the Tribunal on grounds that explain how the novelty of an invention should be assessed.
The court explains: “in order to be included in the state of the art and to be lacking in novelty, the invention must be found in its entirety in a single confirmed prior art, with the same constituting elements, in the same form, the same configuration, with the same function, aspiring to the same technical result”.
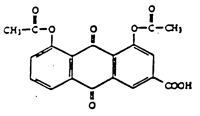
In the present case, only claim 14, which did not relate to the product’s manufacturing process (claims 1 to 13) but to the pharmaceutical product itself, was at issue and drafted as follows: “pharmaceutical preparation containing diacetylrhein having less than 20 ppm of aloe-emodin components together with conventional pharmaceutical carriers and auxiliary supports”.
Was this product new or, on the contrary, did it already form part of the state of the art?
The appellants themselves did not claim that the active diacetylrhein substance was new. Diacetylrhein and its therapeutic antiarthritic value formed part of the state of the art prior to the patent in question and were namely exposed in the invention by Charles Friedmann that gave rise to the United States Patent 4,244,968, filed on 1 March 1977 by Propter, issued on 13 January 1981, concerning, in short, “1,8-Dihydroxy- and 1,8-diacetoxy anthraquinones (i.e. diacetylrhein) and derivatives thereof are used to treat the symptoms of arthritis”.
The only novel element claimed consisted in the slighter aloe-emodin content allowing for not only a more limited use in the acute phase of the illness, but also for a long-term use with no toxicity risk.
However, the Cour d’Appel, like the Tribunal, considered that even in that form of a higher purity, with a reduced aloe-emodine content (less than 20 ppm), the claimed product already formed part of the state of the art. The prior US patent No. 4,244,968 mentioned two other processes for the synthesis of diacetylrhein (Examples 1 and 13) which, implemented according to the general knowledge of the person skilled in the art in the field considered at the date of patent EP 0 520 414, already led to a diacetylrhein containing less than 20 ppm of aloe-emodin.
Experiments carried out in order to achieve the synthesis of diacetylrhein according to the indications in Example 1 and Example 13 of the American Propter Friedmann patent and by using the general knowledge of the person skilled in the art in the relevant technical field, had led to a diacetylrhein containing 0.73 ppm and 1.44 ppm of aloe-emodin components, i.e. an aloe-emodine content of less than 20 ppm as presented in claim 14 of the patent.
And, in the opinion of the court, it was no use to contest the conclusions of those experiments by claiming that the experimenter had used very pure and very expensive elements, since the assessment of novelty as a condition for the patentability of an invention does not require an evaluation of its economic profitability, but only the determination of whether or not it is comprised in the previous state of the art. In the present case, firstly, the American Propter Friedmann patent, as drafted, did not require the use of raw and impure elements and therefore did not exclude the use of purified elements and secondly, by using the general knowledge of the technical field considered at the time, the person skilled in the art was able to purify those elements.
These are the reasons why the Cour d’Appel considered that the American Propter Friedmann patent, which disclosed the compound named diacetylrhein, had made this product available in all degrees of purity and deprived of novelty the pharmaceutical product presented in claim 14 of European patent No. 520 414.
Original French decision.
English translation.
Author: Nicolas Bouche, Head Legal Research and Literature, Véron & Associés, Paris, France
________________________
To make sure you do not miss out on regular updates from the Kluwer Patent Blog, please subscribe here.



This decision was also reported here:
http://jurisprudencebrevets.blogspot.com/2010/09/ou-lon-parle-nouveau-de-purete.html