Boehringer is the holder of the European patent No. 0589 874, filed on 21 June 1991 and entitled “use of (S)(+)-2-ethoxy-4-[N-[1-(2-piperidinophenyl)-3-methyl-1-butyl]aminocarbonylmethyl]-benzoic acid for the preparation of a long-lasting antidiabetic medicament”. On 22 August 2008, Hexal AG and Sandoz brought proceedings against Boehringer before the Tribunal de Grande Instance of Paris for the invalidity of the claims of the French designation of the patent for lack of novelty or at the very least lack of inventive step, pursuant to the provisions of Articles 138-1-a), 52-1, 54-2 and 56 of the European Patent Convention. In its decision of 7 May 2010, the Tribunal de Grande Instance of Paris supports the claimants and holds the French designation of the European patent invalid for lack of inventive step of claims 1 and claims 4 to 7 and for lack of novelty of claims 2 and 3. The key ruling of this decision is that in the present case, the fact that Boehringer separated and tested the two enantiomers of a chiral molecule having an already-know therapeutic effect in order to identify the active enantiomer, to evaluate its specific therapeutic effect and finally to recommend through the patent its use for an identical but better-quality therapeutic effect, involved no inventive step.
This is not the first French decision holding invalid a patent on an isolated enantiomer. The Tribunal de Grande Instance of Paris in a decision of 6 October 2009 (Teva v. Sepracor) has already held invalid such a patent for insufficiency. In Belgium, the first enantiomer decision was rendered on 11 December 2009 by the Commercial Court of Antwerp which invalidated the patent for lack of inventive step with a reasoning very similar to the present case. Another parallel can be drawn between the present decision and the famous decision H. Lundbeck A/S v. Generics (UK) Ltd. of the Court of Appeal on 10 April 2008 at least as for the novelty. The obviousness was also controled by the Court but about an amino diol method specified in claim 6 and not really about the (+)enantiomer as such. Therefore, it seems rather difficult to compare the two decisions on this specific issue.
The court first carefully explains the specific nature of the molecule, subject-matter of the patent. A chiral molecule has a specific atomic structure in which an atom, generally carbon, is attached to four atoms or groups of atoms all different from one another. The molecule exists in two different forms that are mirror images of each other, non-superposable on each other. These two chiral molecules, which are isomers because their atomic composition is identical, but also stereoisomers (the (R)/(S) nomenclature describes this stereochemical configuration of the carbon atom) because their spatial arrangement is different (the atomic arrangement of the one being the non-superposable mirror image of the other) are known as enantiomers (the (+)enantiomer and the (-)enantiomer).
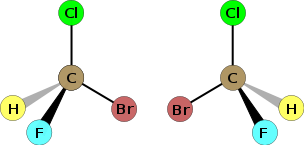
The synthesis of chiral compounds through conventional methods generally provides a racemate, i.e. a mixture of the two enantiomers in equal amounts. However, it must be underlined that in a synthesis chiral substance used as a medicament, of the two possible enantiomers generally only one has the required pharmacological properties. In a best-case scenario, the other enantiomer is merely a useless loading when it is not toxic or even teratogenic. It is not advantageous to the patient to consum a medicament in its racemic form; on the contrary, the therapeutic effect will necessarily be increased with a enantiomerically pure compound (so-called enantiopure), composed only of the active enantiomer, itself alone producing the therapeutic effect that was appreciated in the racemic mixture. The subject-matter of the patent in dispute is precisely the claim of the sole enantiomer of a chiral molecule for an already-known therapeutic administration for treating diabetes mellitus. While the racemic mixture (2-ethoxy-4-[N-[1-(2-piperidinophenyl)-3-methyl-1-butyl]aminocarbonylmethyl]benzoic acid), which provides treatment for diabetes mellitus, had already been previously patented for such a therapeutic administration (European patent No. 0147 850 filed on 27 December 1984 and European patent No. 0207 331 filed on 10 June 1986 for two new solid forms of the racemic mixture), Boehringer has identified the active enantiomer of this racemix mixture (in this case the (S)(+)enantiomer) and has patented the use of this enantiomer for the same therapeutic administration underlining that this enantiopure has a better-quality therapeutic effect (the improvement being even unexpected, according to Boehringer) than the racemic mixture.
First, the court decides that claim 1 of the patent in dispute is not invalid for lack of novelty because the European patents No. 0147 850 filed on 27 December 1984 and No. 0207 331 were not novelty-destroying prior art documents. While these two prior patents both claim the use of the racemic mixture of 2-ethoxy-4-[N-[1-(2-piperidinophenyl)-3-methyl-1-butyl]aminocarbonylmethyl]benzoic acid for treating diabetes mellitus, none of the prior patents studied the specific effect of the S(+)enantiomer compared with the racemate from which it is extracted.
However, the court supports the claimants and holds the French designation of the European patent invalid for lack of inventive step of claims 1 and claims 4 to 7 and for lack of novelty of claims 2 and 3.
Claim 1 relates to “Use of (S)(+)-2-ethoxy-4-[N-[1-(2-piperidinophenyl)-3-methyl-1-butyl]aminocarbonylmethyl]-benzoic acid as active substance, or of a physiologically acceptable salt thereof, in the preparation of a long-term antidiabetic agent, characterised in that, compared with double the single dose in the administration of a racemate, unnecessarily high and long-lasting substance loading is avoided, as a result of which substantially lower levels of active substance in the plasma are obtained which go beyond the normal advantage of halving the dose in the administration of enantiomers”. In the court’s opinion, the claimed invention was obvious to a person skilled in the art through his knowledge of the state of the art at the filing date of the patent application. On the one hand, the person skilled in the art is in the present case “an organic chemist specialised in organic molecular synthesis for therapeutic purposes, informed of the structure and activity of the pharmaceutical active substances still in development and already used, and of the preparation of agents containing such active substances, and who is part of a team of experts involved and informed of the discovery of new active substances and of their development, this team also including, given the objective, pharmacologists, medical doctors and veterinary surgeons involved in clinical research as well as chemists and analysts, sought to resolve the problem posed, namely: to prepare an antidiabetic agent suitable for long-term treatment using an appropriate active substance, this antidiabetic agent having beneficial pharmacological properties with respect to the state of the art”. On the other hand, at the filing date of the patent application, as a result of different published articles, it became clear that the scientific community was generally aware of the fact that a chiral therapeutic molecule often only has one active enantiomer from a therapeutic point of view, the other being possibly inactive, toxic, even teratogenic, or contributing to side-effects. Consequently, the person skilled in the art was encouraged and prompted by this literature to separate the enantiomers of a chiral therapeutic molecule used as a medicament in its racemic form in order to test the specific therapeutic effect of each enantiomer and to determine if necessary that the therapeutic activity of the active principle is enantioselective. Accordingly the person skilled in the art, aware of the literature published on the issue of whether the therapeutic activity of the active principle is enantioselective, and who wanted to prepare a suitable antidiabetic agent for long-term treatment by using an appropriate active substance, this antidiabetic agent having beneficial pharmacological properties with respect to the state of the art, had no prejudice to overcome to study the effects of the two enantiomers of 2-ethoxy-4-[N-[1-(2-piperidinophenyl)-3-methyl-1-butyl]aminocarbonylmethyl]-benzoic acid, the racemate of which proved to be very promising. Furthermore, the person skilled in the art would have undertaken the separation of the enantiomers from 2-ethoxy-4-[N-[1-(2-piperidinophenyl)-3-methyl-1-butyl]aminocarbonylmethyl]-benzoic acid by using the method described in the prior art document EP 850 to separate compound E, (S)(+)enantiomer in the racemate which is compound B. And the court also dismisses Boehringer’s argument according to which inventive step would result from the unexpected pharmacological properties of the isolated enantiomer. While an effect considered as unexpected may indeed involve an inventive step, a claim lacks inventive step in the cases in which the person skilled in the art would arrive in an obvious manner at a result which corresponds to the terms of a claim by taking into consideration the state of the art, considering that he could expect the combination of the teachings of the documents comprised in the state of the art to provide an advantage, independently from the fact that a possibly unexpected additional effect is obtained. This was effectively the case in the present situation since the additional and unexpected effect obtained according to which “compared with double the single dose in the administration of a racemate, unnecessarily high and long-lasting substance loading is avoided, as a result of which substantially lower levels of the active substance in the plasma are obtained which go beyond the normal advantage of halving the dose in the administration of enantiomers”, constitutes a mere additional effect occuring spontaneously over the course of the studies suggested by the state of the art, which does not confer any inventiveness.
The court also holds invalid claims 2 and 3 which relate to the use of the active enantiomer of claim 1 with a high degree of purity (ee = 95 or 98) for lack of novelty. A known compound does not acquire novelty simply from the fact that it is prepared in a purer form because it is common practice in synthesis organic chemistry for the person skilled in the art to continue to purify a chemical compound obtained according to a particular process until it reaches the degree of purity required, so that a document disclosing a chemical compound makes this product available within the meaning of Article 54 of the European Patent Convention, in all the degrees of purity. In the present case, the prior art documents EP 850 and EP 331 disclosing the racemate, composed in equal amounts of both enantiomers, also disclosed in consequence its two enantiomers in all the possible degrees of purity.
Finally, the court held invalid claims 4 to 7 which each relate to the use of the enantiomer of claim 1 with a different single dose, without corroborating experimental results or any pharmaceutical effect being associated to it. These so-worded claims cannot be sufficient to show an inventive step.
_____________________________
To make sure you do not miss out on regular updates from the Kluwer Patent Blog, please subscribe here.
Kluwer IP Law
The 2022 Future Ready Lawyer survey showed that 79% of lawyers think that the importance of legal technology will increase for next year. With Kluwer IP Law you can navigate the increasingly global practice of IP law with specialized, local and cross-border information and tools from every preferred location. Are you, as an IP professional, ready for the future?
Learn how Kluwer IP Law can support you.


